In 1977, Engleman et al. described pulmonary hyalinizing granuloma (PHG) as a separate entity.1 This disease is rare and is radiologically characterized by multiple and often bilateral nodules with no preferential localization.1,2 Its etiology and pathogenesis remain unclear, and a definitive diagnosis is provided by histopathological study of the lesions describing hyalinized lamellar collagen bundles surrounded by plasma cells, lymphocytes, and histiocytes. This disease usually evolves with a benign course with nodules slowly increasing in size over years. Specific treatment is not usually required, although corticosteroids have demonstrated some efficacy.1–3
The etiology of PHG is not well established, and no relation to occupational exposure has been identified. Due to the frequent association with infectious, autoimmune, and tumoral diseases, an abnormal immune reaction has been proposed to explain the development of PHG.1,3,4 In the case in the present report, we describe an uncommon association between PHG and a lymphoproliferative disorder. We found only four similar reports of associations with multicentric Castleman disease5,6; diffuse lymphocytic lymphoma of the abdomen7; and pulmonary small lymphocytic lymphoma8. The ages of these patients ranged between 43 and 50 years, and the patients were predominantly male (3 males/1 female). All patients presented with multiple and bilateral pulmonary nodules, and diagnosis was made through video-assisted thoracoscopic lung biopsy in three cases and by post-mortem examination in one case. Chemotherapy schemes targeted at lymphoproliferative disease which included corticosteroid were initiated in three cases. In two of these cases, there was a reduction in the lung nodule dimensions.
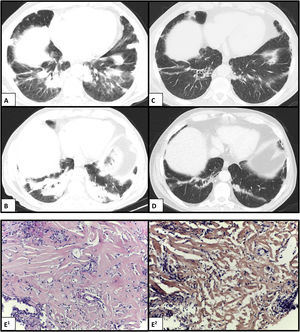
We report a case of a 69-year-old man, former smoker (70 pack-years), with occupational exposure to dust and fumes from metallurgical casting and contact with birds, who presented to the emergency department with a 6-month history of progressive dyspnea. He reported mild fever, anorexia, and weight loss (10 kg over the prior 3 months). A physical examination revealed inspiratory crackles in the lower lungs. Chest computed tomography (CT) scan showed bilateral peripheral consolidation areas, mainly in the lower lobes but also involving the middle lobe and lingula (Fig. 1 — A and B); the CT also revealed hepatosplenomegaly. Laboratory data demonstrated normocytic and normochromic anemia, and elevated levels of C-reactive protein and erythrocyte sedimentation rate. An autoimmunity study was negative. No endobronchial lesions were detected in the bronchoscopy. In the bronchoalveolar lavage, the total and differential cell counts revealed lymphocytic (47%) and neutrophilic (7.4%) alveolitis, which was negative for microorganisms and malignant cells. An initial pulmonary function test revealed moderate restrictive ventilatory alteration with a forced expiratory volume in one second (FEV1) 2.06 L (72.8% of the predicted value), forced vital capacity (FVC) 2.42 L (65.6%), preserved FEV1/FVC ratio, a total lung capacity (TLC) 4.51 L (68.5%), and carbon monoxide transfer factor (TLCO) 52%. A CT-guided transthoracic core biopsy was subsequently performed, and hyalinized lamellar collagen tissue surrounded by lymphoplasmacytic infiltrates was reported on histological examination (Fig. 1E1). No evidence of neoplastic cells was found, and acid-fast, fungal, and Congo red stains were all negative (Fig. 1E2). These findings were consistent with a diagnosis of PHG. The patient was started on oral corticosteroid therapy (deflazacort in an equivalent dose of 0.5 mg/kg/day of prednisolone, within a weaning scheme) and demonstrated partial clinical and functional but not radiological improvement.
Chest CT scan showing bilateral peripheral consolidation areas in the lower lobes before (A and B) and after (C and D) complete chemotherapy scheme. (E1) Histology showing hyalinized lamellar collagen tissue surrounded by lymphoplasmacytic infiltrates (hematoxylin and eosin, ×200); and (E2) negative Congo red stain (CR, ×200).
Throughout the investigation, an indolent non-Hodgkin’s lymphoma (NHL) was incidentally diagnosed, supported by a bone marrow biopsy. The clinical case was, therefore, discussed in a multidisciplinary team, and with PHG hypothesized as a paraneoplastic manifestation of hematological disease, a decision to start NHL treatment was made. After completing eight cycles of chemotherapy (rituximab, cyclophosphamide, vincristine, and prednisolone), the patient had significant clinical, functional, and radiological improvement (Fig. 1 — B and D). At the time this report was written, the patient was asymptomatic and functional with FEV1 2.88 L (106.5%), FVC 3.66 L (103.1%), TLC 5.83 L (89.7%), and TLCO 68%. No residual disease was found on further bone marrow tests.
In summary, PHG is a rare entity with nonspecific symptoms and slow progression. Although isolated or multiple nodular lesions are the most frequent findings, PHG can occaisonally appear as lung parenchymal infiltration or consolidation with irregular or indistinct borders, such as in this case.3 In patients showing a rapid course and significant functional impairment, corticosteroid treatment may be attempted, despite the unclear efficacy of this approach. Due to the frequent association with underlying diseases, a careful investigation should be performed for therapeutic and prognostic implications.
Ethical disclosuresThere are no personal details of patient in any part of the paper and in any supplementary materials (including illustrations).
FundingNo funding.
Conflicts of interestsThe authors have no conflicts of interest to declare.
The authors present a clinical case of a rare disease with atypical radiological presentation, and an uncommon association between Pulmonary Hyalinizing Granuloma and a lymphoproliferative disorder.