Hypoventilatory respiratory failure is the most common cause of death and respiratory morbidity in patients with amyotrophic lateral sclerosis (ALS).1 Non-invasive ventilation (NIV) prolongs survival and improves quality of life; however, its benefits depend on the optimization of both ventilation and adherence.1,2 Upper motor neuron involvement and impairment of bulbar function are associated with upper airway (UA) spasticity and sometimes preclude NIV support in ALS patients.2
NIV’s acceptance and adherence can be influenced by the site of disease onset, NIV settings and comfort, presence of asynchrony, aggressiveness of disease, ALS phenotype, psychological defense to clinical status,3 and types of mask interface.1,4 However, this subject remains debatable and is inconclusive, requiring further exploration and strategies, as marked by Vitacca et al.3
Telemonitoring could be one of the strategies to obtain greater adherence, offering information on the presence of leaks, continuous monitoring of breathing, continuous pulse oximetry, and even capnography monitoring, according to Angelucci et al.5 In addition, physiological changes such as hypoventilation, hypoxemia, hypercapnia, and sleep disorders can be monitored.5 Home care ventilators can have a built-in monitoring system. It enablesn earlier effective intervention and is a promising solution both from the technical and economic points of view, as it improves the quality of the care provided.5
ALS patients may exhibit weaknesses of the tongue and pharyngeal muscles, promoting UA obstruction, especially when positive inspiratory pressure (PIP) is applied.2,4,6 During insufflation, especially in bulbar ALS patients, adduction of supraglottic laryngeal structures can occur. This compromises airflow and, when posterior displacement of the base of the tongue is associated, increases hypopharynx obstruction.2 Pharyngeal collapse caused by abrupt inspiratory-expiratory pressure reduction are other potential mechanism of NIV’s influence on the upper airway.2,4,6 These changes can influence ventilatory parameters because positive pressures may compromise the laryngeal inlet during insufflation due to a retroflex movement of epiglottis2, narrowing the glottis, decreasing the tidal volume delivered6, and affecting adherence to the NIV.3
Oronasal masks may be preferred because of better oral leak control, but potential disadvantages include claustrophobia, aspiration risk, increased dead space ventilation, and relevant compromises of eating and speaking.4 Other potential mechanisms related to the oronasal mask that can happen even in patients with elevated EPAPs (>12cmH2O) are the collapse of the soft palate, backward movement of the epiglottis, or tongue base obstruction, with a consequent reduction in the retroglossal space, as reported in a review by Conde et al.2 Nasal masks are usually preferred for convenience, but bear the risk of insufficient ventilation if mouth leaks occur. These are considered advantageous for positive airway pressure therapy due to better tolerability and sleep quality, lower pressure needs, and increased adherence.4
Videoendoscopy2 and videofluoroscopy7 are used to assess UA spaces and anatomo-functional changes. UA videoendoscopy during ventilation titration,2 while implying some degree of invasiveness, may be useful in detecting non-predictable problems and in guiding therapeutic decisions, such as replacement of the mask interface, extending the use of NIV, correcting the observed collapse, and selecting the best time and patients to perform a tracheostomy. There are no standardized videoendoscopic evaluation protocols for assessing UA spaces during NIV use in patients with ALS, but these strategies could extend NIV support to another level, as pointed out by Conde et al.2 There are no reports in the literature on the use of videofluoroscopy to assess UA during NIV use.
Vrijsen et al.6 reported a case of a patient with ALS who showed intermittent posterior displacement of the tongue by PIP when using an oronasal mask, inducing obstructive events in the UA and resulting in sleep fragmentation and decreased efficiency of NIV. These findings can possibly be generalized in the ALS clinical setting.4,6
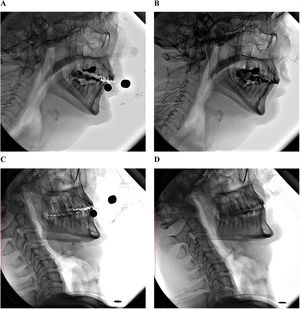
To illustrate this, we present two cases of ALS patients using NIV while awake, monitored through videofluoroscopy (Video S1). The aim is to demonstrate the qualitative differences in the UA when using NIV with two different mask interfaces. Clinical data, ventilation details, and pharyngeal area measurements (squared C2–C4), as proposed by Stokely et al.,7 are shown in Fig. 1.
Clinical data, ventilation details and pharyngeal area measurements. Patient 1 is a 64-year old male, diagnosed with ALS 1.5 years ago, ALSFRS-R of 32 points, Fuctional vital capacity of 69%, ventilated with a ResMed Stellar 150 (ET mode; IPAP 12 cmH2O; EPAP 4 cmH2O; Inspiratory time 0.7-1.2s; Respiratory frequency: 16 bpm; Cycle: medium; Rise time: 250 msec; Fall time: 100 msec). Using an oronasal mask RedMed AirFit F20 he presented a pharyngeal area (squared C2-C4, as proposed by Stokely et al.) of 8.91 cm2 (A) and with an intranasal mask ResMed AirFit P10 of 8.74 cm2 (B). Patient 2 is a 48-year old male, diagnosed with ALS 8 years ago, ALSFRS-R of 6 points, Functional vital capacity of 12%, ventilated with a ResMed Stellar 150 (ET mode; IPAP 17 cmH2O; EPAP 6 cmH2O; Inspiratory time: 0.8-1.5s; Respiratory frequency: 16 bpm; Cycle: medium; Rise time: 300 msec; Fall time: 200 msec). Using an oronasal mask ResMed AirFit F20 he presented a pharyngeal area (squared C2-C4) of 2.29 cm2 (C) and with an intranasal mask ResMed AirFit P10 of 6.21 cm2 (D).
During the use of the oronasal mask, the patient with 1.5 years of disease presented a slight increase in the UA spaces, while the patient with an 8-year history presented an important reduction in the pharyngeal area. This finding is probably related to bulbar weakness, which is part of the natural ALS evolution. This raises the need to test different interfaces according to the severity of the disease to investigate adherence and improvement of ventilatory parameters.
Disparities can be seen with regard to UA area and position of the mandible, tongue, soft palate, and hyoid bone, considering that ALS is a clinically heterogeneous disease. Non-invasive methods to assess UA during NIV use, such as videofluoroscopy, should be performed in patients with low adherence to explore additional causal factors. Future studies must be conducted to quantitatively assess these differences, especially with respect to ALS severity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Source of fundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.