Chronic Critical Illness (chronic CI) is a condition associated to patients surviving an episode of acute respiratory failure (ARF). The prevalence and the factors associated with the development of chronic CI in the population admitted to a Respiratory Intensive Care Unit (RICU) have not yet been clarified.
MethodsAn observational prospective cohort study was undertaken at the RICU of the University Hospital of Modena (Italy). Patients mechanically ventilated with ARF in RICU were enrolled. Demographics, severity scores (APACHEII, SOFA, SAPSII), and clinical condition (septic shock, pneumonia, ARDS) were recorded on admission. Respiratory mechanics and inflammatory-metabolic blood parameters were measured both on admission and over the first week of stay. All variables were tested as predictors of chronic CI through univariate and multivariate analysis.
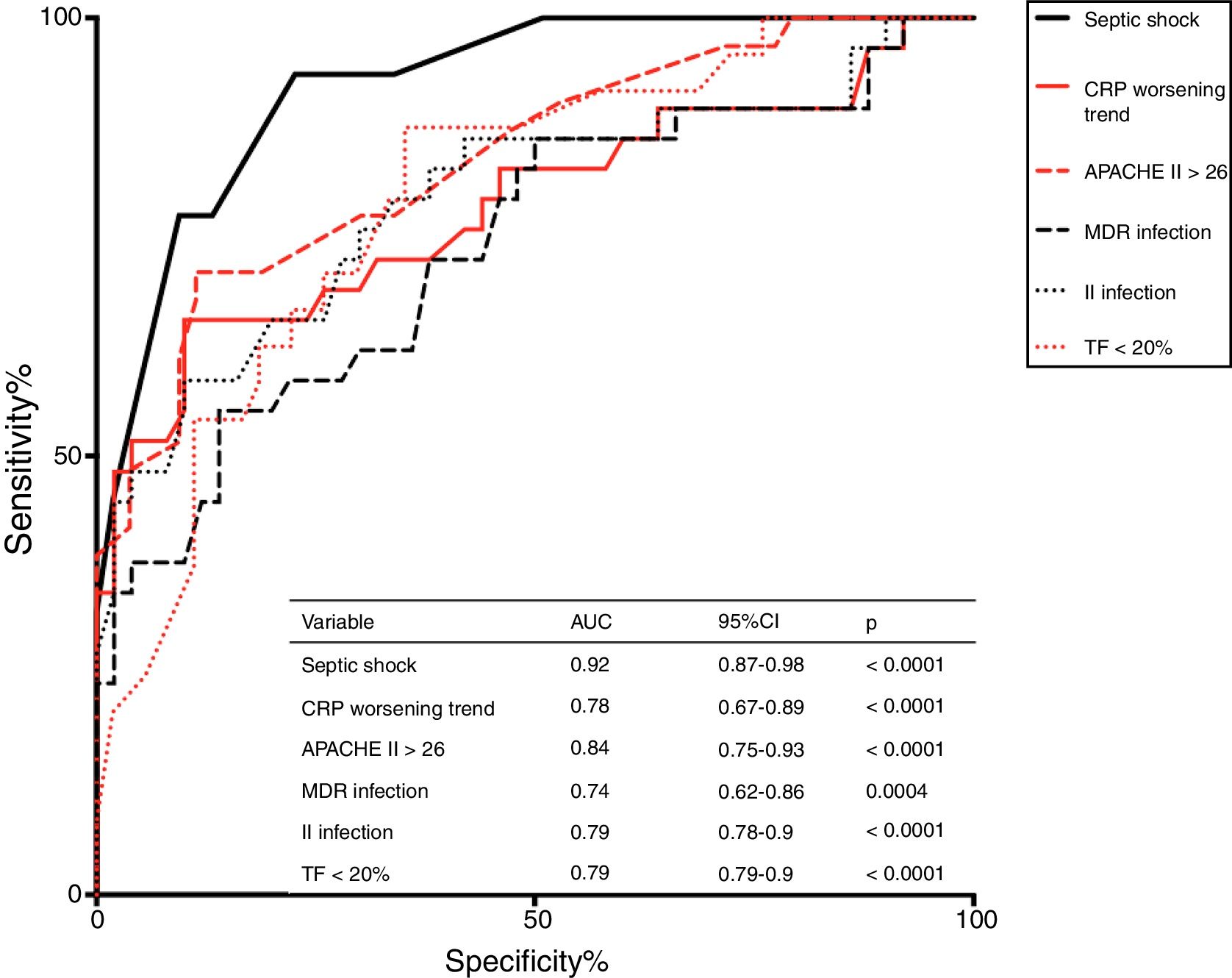
ResultsChronic CI occurred in 33 out of 100 patients observed. Higher APACHEII, the presence of septic shock, diaphragmatic dysfunction (DD) at sonography, multidrug-resistant (MDR) bacterial infection, the occurrence of a second infection during stay, and a C-reactive protein (CRP) serum level inceasing 7 days over admission were associated with chronic CI. Septic shock was the strongest predictor of chronic CI (AUC = 0.92 p < 0.0001).
ConclusionsChronic CI is frequent in patients admitted to RICU and mechanically ventilated due to ARF. Infection-related factors seem to play a major role as predictors of this syndrome.
In recent years, life support techniques have improved the outcomes of patients with acute respiratory disorders, but have also led to the emergence of a population of survivors even though dependent on mechanical ventilation (MV), the so called “chronically critical ill-chronic CI”.1
In western countries, chronic CI is rapidly growing and it is associated with increased number of days in the intensive care unit (ICU), prolonged hospitalization in post-acute (weaning) centers, and poor prognosis in the long term.2 Around 50% of patients developing chronic CI die within 6 months following discharge from ICU, while only 10% of survivors return home with autonomy.3,4
The clinical features of these patients are consistent enough to define chronic CI as a syndrome characterized by neuroendocrine changes, alteration of body composition, neurological modifications, malnutrition and muscle wasting.5–7 However, an exhaustive definition of chronic CI is still not available. A recent consensus definition resulted from the combination of two factors: care received in ICU for at least 8 days, and at least one out of five eligible conditions (MV prolonged >96 h; tracheostomy; sepsis or other severe infections; severe wounds and/or multiple organ failure; ischemic stroke, intercerebral hemorrhage or traumatic brain injury), prolonged MV and tracheostomy were the two reliable indicators of chronic CI occurring in patients with acute respiratory failure (ARF).8,9
Although chronic CI is described in the whole population of patients admitted to ICUs,10–12 there are still no studies focusing on those patients suffering from de novo ARF following conditions with a vigorous local and systemic inflammatory response, such as pneumonia and Acute Respiratory Distress Syndrome (ARDS).13,14
Primary aim of this study was to describe the prevalence and the development of chronic CI in a population of patients with de novo ARF admitted to a specialized RICU. The association between clinical and mechanical features and the development of chronic CI was investigated as secondary outcome.
Materials and methodsStudy populationThis prospective observational cohort study was carried out in a single 6-bed RICU at the University Hospital of Modena (Italy) over a 24-month period (January 2016–January 2018). Written informed consent to participate was obtained from all patients enrolled or their relatives. Approval was obtained from the local ethics committee of Modena (protocol 839/C.E. and 266/16 C.E.), and the trial was registered at clinicaltrials.gov (NCT03851822).
Eligible patients >18 years of age were those consecutively admitted to the RICU due to ARF (hypoxemia or hypoxemia with hypercapnia) requiring MV, and with a stay >8 day. Exclusion criteria were: 1) refractory shock, 2) patient goals of care not consistent with aggressive management, 3) end-stage chronic obstructive pulmonary disease (COPD) requiring home oxygen and/or ventilatory support, 4) interstitial lung disease, 5) neuromuscular disease, 6) chest wall deformities, 7) pregnancy, 8) chemotherapy or radiotherapy during the past 30 days, 9) brain injury on CT scan and/or Glasgow Coma Scale (GCS) score <8, 10) tracheostomy. All patients were treated according to the best current clinical practice by the attending staff, unaware of the study purpose. All patients were provided with mobilization and rotation on a daily basis.
General measurementsDemographics, severity scores, namely the Kelly-Matthay Scale, the Acute Physiology and Chronic Health Evaluation II (APACHE II), the Simplified Acute Physiology Score (SAPS II), and the Subsequent Organ Failure Assessment (SOFA) were recorded at admission. Presence of ARDS, pneumonia or septic shock was assessed at baseline whereas pre-existing comorbidities were reported through the Charlson Comorbidity Index; concomitant COPD had to be confirmed by both smoking habit, past medical history, non-reversible obstruction at spirometry performed in the year before. Hospitalizations in the previous 6-month were also reported.
Arterial blood gases (PaO2, PaCO2), pH, and PaO2/FiO2 ratio were recorded at baseline; blood procalcitonin, glucose, albumin, creatinine and lactate were measured at baseline and after 1, 2 and 7 days following admission. Multidrug-resistant (MDR) microorganism colonization and infection, and the onset of a second infection during RICU stay were recorded.
Lung mechanicsA subset of patients under MV as divided into two groups according to the ventilatory mode (assisted spontaneous breathing — ASB, and controlled ventilation-CMV) was subjected to measurement of lung mechanics.
Patients in the ASB group underwent esophageal pressure (Pes) and transpulmonary pressure (PL) monitoring performed through a nasogastric tube (NutriVent nasogastric polyfunctional catheter; SIDAM, Mirandola, Italy) with a pressure transducer (OptiVent monitor, SIDAM) and according to a standard procedure.15,16 PL was calculated as airway pressure (Paw) – Pes. In order to avoid using absolute values for Pes and PL, we always referred to ΔPes and ΔPL from the end-expiratory level, respectively. The waveforms of Paw, Pes and airflow were continuously recorded using a data acquisition system (PowerLab; AD Instruments, Colorado Springs, CO, USA) at a sampling frequency of 100 Hz for offline data analysis.17
Patients in the CMV group, underwent ventilation set in control mode with a Vt = 6 ml/kg and positive end-expiratory pressure (PEEP) adjusted on the basis of the incremental FiO2/PEEP combinations.18 Measurements of static respiratory mechanics were performed after 30 min of constant flow during MV. The values of pressure were obtained during baseline ventilation with an airway occlusion at the end of inspiration pressing the end-inspiratory hold, until reaching a plateau pressure, and subsequently performing a similar procedure with an end-expiratory occlusion. During the procedure, occlusions that did not produce a clear plateau were discarded. Airway driving pressure (ΔPaw) was defined as the end-inspiratory plateau pressure (Pplat) — positive end-expiratory airway pressure (PEEPtot). All these measurements were performed within 24 h from MV start.
Diaphragmatic functionUltrasound (US) of the diaphragm was assessed on both sides at admission during spontaneous breathing. Motility of the diaphragm was assessed at the bedside in the semi-recumbent position by a B-mode US device (GE Vivid 7; GE Healthcare Life Sciences, Helsinki, Finland) connected to a 7–12 MHz linear probe as previously reported.19 Thickening fraction (TF) during spontaneous breathing19 was calculated as: ΔTdi = (end-inspiration Tdi – end-expiration Tdi/ end-expiration Tdi) × 100. The best value of three measurements was taken as representative of the diaphragm function and then recorded for analysis.
Definitions and outcomesDiaphragm dysfunction (DD) was defined according to the presence of TF bilaterally lower than 20%.19
Survivors with both hospital stay >8 days and with tracheostomy due to a need of MV > 21 consecutive days for at least 6 h/day were defined as having chronic CI.1 The prevalence of chronic CI was the primary aim of this study.
According to the Berlin definition, patients were considered as having ARDS if they had: [1] acute respiratory failure not fully explained by cardiac failure or fluid overload; [2] bilateral opacities consistent with pulmonary edema on the chest radiograph or the computed tomography scan; and [3] onset within 1 week after a known clinical insult or new/worsening respiratory symptoms.20 Septic shock was present according to the onset of sepsis with requirement of vasopressor to maintain a mean arterial pressure ≥65 mmHg and serum lactate level >2 mmol/L (>18 mg/dL) in the absence of hypovolemia.21 Multi-Drug-Resistant (MDR) infection was defined as the presence of sepsis with blood isolation of a MDR bacterial agent. The onset of a second infection was defined by the occurrence of clinical, radiological and microbiological signs of infection during RICU stay (after at least 2 days of stability when recovering from the first episode). The association between chronic CI and demographics, inflammatory and metabolic blood parameters (C-reactive protein-CRP, sepsis and septic shock, infection and MDR bacterial infection), and respiratory mechanics (transpulmonary and driving pressure) on admission and/or their trends between days 1 and 7, were considered as secondary outcomes of the study.
Statistical analysisThe statistical package GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Initially, we performed a power test (α = 0.05, power 80%) considering a prevalence of up to 20% of patients undergoing MV for >48 h and/or tracheostomy in the critical care area,7 and an average annual mortality rate of 24% among patients admitted to RICU and requiring MV. A sample size of 127 patients was required to confidently perform analysis on the pre-specified primary scope of our study.
The considered variables were investigated through univariate analysis and contingency table analysis for relative risks to detect the possible risk factors associated with chronic CI once in RICU.
Significant variables then entered a multivariate regression analysis, with a backward stepwise method to exclude non-significant variables from the model. Independent variables able to predict chronic CI at the multivariate test were then entered intoa ROC analysis.
A p-value lower than 0.05 was considered to be statistically significant.
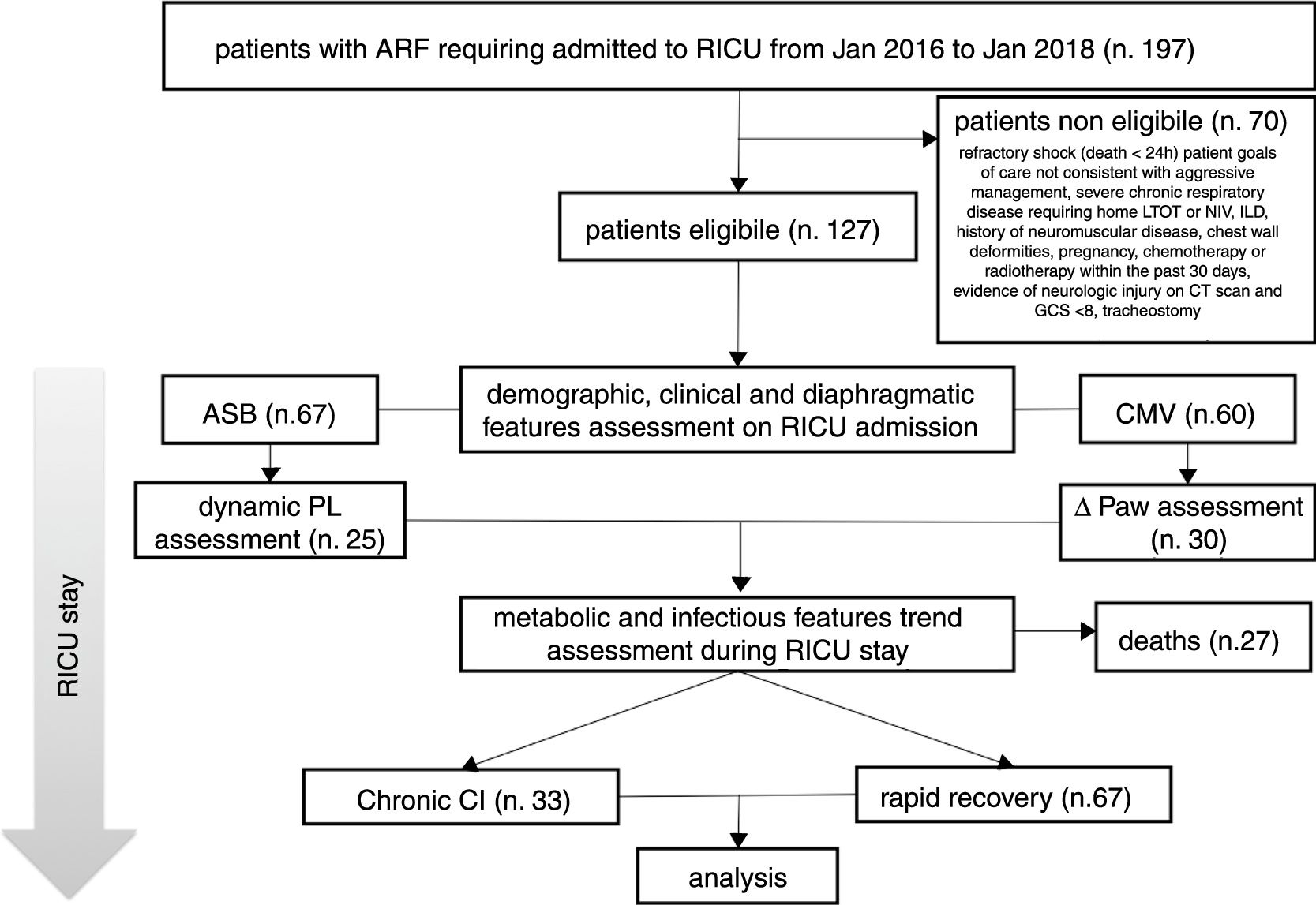
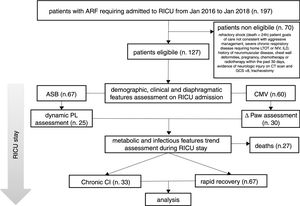
ResultsIn the period considered, 127 patients were found to be eligible; 27 out of the 127 subjects died in RICU and were excluded from the analysis (see Fig. 1).
Description of the study population.
ARF = acute respiratory failure, MV = mechanical ventilation, RICU = Respiratory Intensive Care Unit, LTOT = Long Term Oxygen Therapy, NIV = Non Invasive Mechanical Ventilation, ILD = Interstitial Lung Disease, CT = Computed Tomography, GCS = Glasgow Coma Scale, ASB = Assisted Spontaneous Breathing, PL = Transpulmonary Pressure, CMV = Controlled Mechanical Ventilation, Paw = Driving Pressure, Chronic CI = Chronic Critical Illness
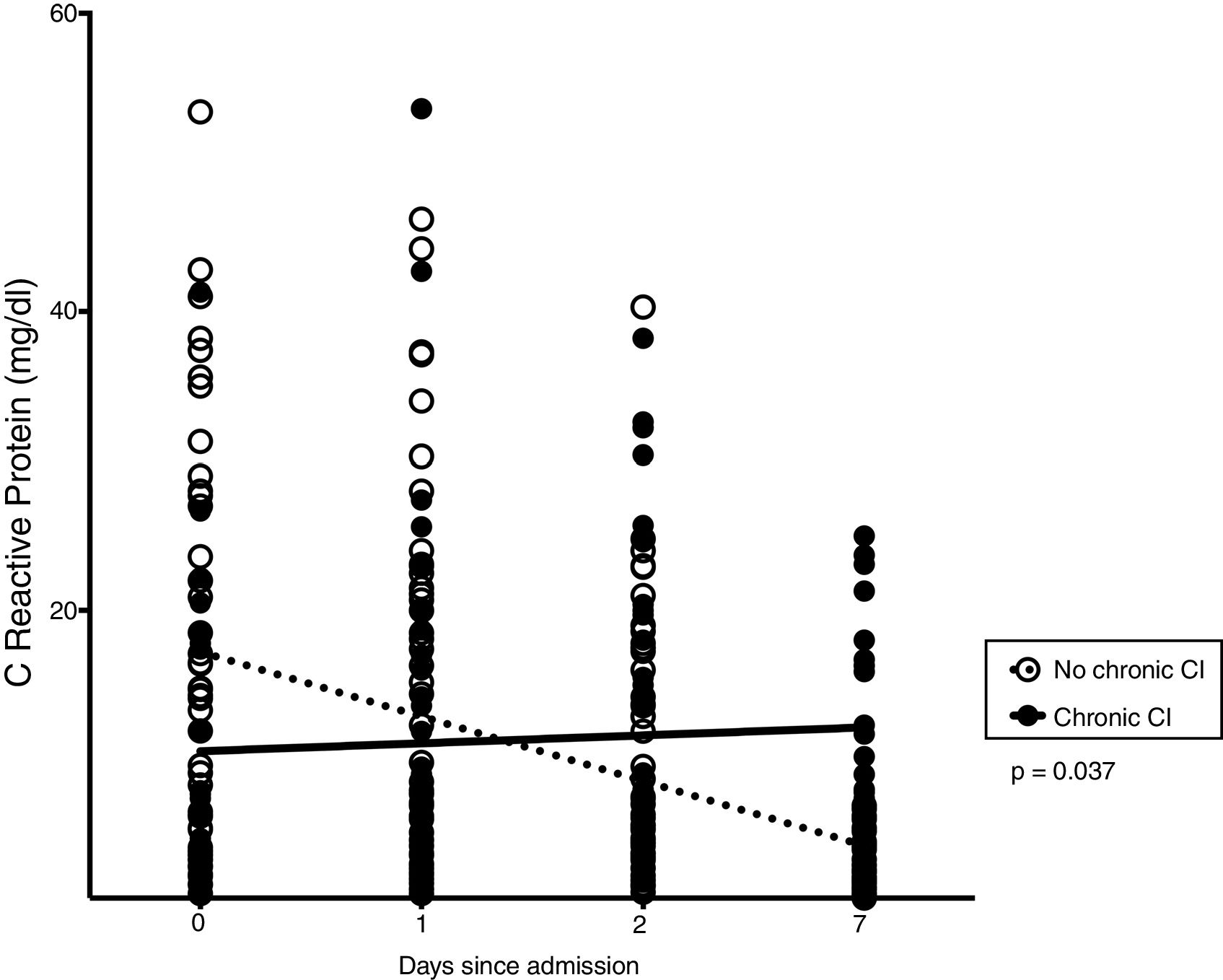
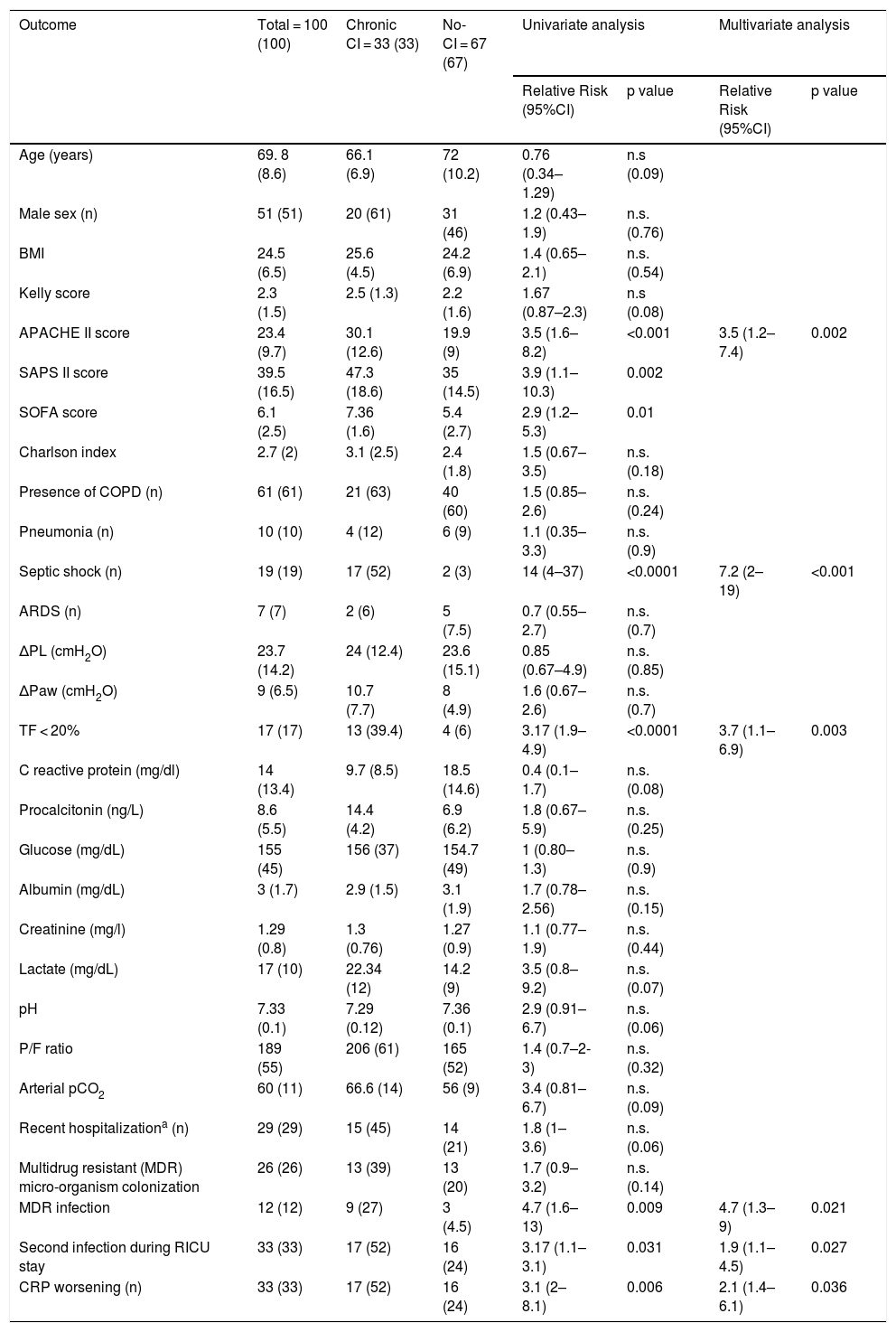
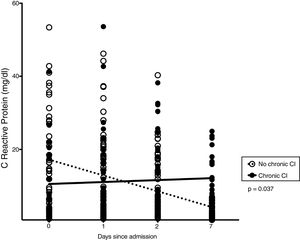
Thirty-three patients (33%) developed chronic CI during stay in RICU. Table 1 shows demographics, biochemistry, clinical and physiological characteristics of patients developing chronic CI (n = 33) as compared with others (n = 67). Causes of de novo ARF included acute exacerbation of COPD (n = 45), septic shock (n = 19), pneumonia (n = 10), pulmonary embolism (n = 10), ARDS (n = 7 [pulmonary infection driven n = 5, extra-pulmonary infection driven = 2]), acute exacerbation of asthma (n = 5), lung inhalation (n = 4). In the subset of patients undergoing lung mechanical assessment, ΔPL and ΔPaw were not associated with chronic CI. The trend of CRP levels, but not other blood parameters, over 1-week from admission was different when comparing patients with or without chronic CI (p = 0.037, Fig. 2).
Demographics, clinical and physiological variables in the study population, univariate and multivariate analysis of variables as potential risk factors for developing critical care illness in the population in this study.
| Outcome | Total = 100 (100) | Chronic CI = 33 (33) | No-CI = 67 (67) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| Relative Risk (95%CI) | p value | Relative Risk (95%CI) | p value | ||||
| Age (years) | 69. 8 (8.6) | 66.1 (6.9) | 72 (10.2) | 0.76 (0.34–1.29) | n.s (0.09) | ||
| Male sex (n) | 51 (51) | 20 (61) | 31 (46) | 1.2 (0.43–1.9) | n.s. (0.76) | ||
| BMI | 24.5 (6.5) | 25.6 (4.5) | 24.2 (6.9) | 1.4 (0.65–2.1) | n.s. (0.54) | ||
| Kelly score | 2.3 (1.5) | 2.5 (1.3) | 2.2 (1.6) | 1.67 (0.87–2.3) | n.s (0.08) | ||
| APACHE II score | 23.4 (9.7) | 30.1 (12.6) | 19.9 (9) | 3.5 (1.6–8.2) | <0.001 | 3.5 (1.2–7.4) | 0.002 |
| SAPS II score | 39.5 (16.5) | 47.3 (18.6) | 35 (14.5) | 3.9 (1.1–10.3) | 0.002 | ||
| SOFA score | 6.1 (2.5) | 7.36 (1.6) | 5.4 (2.7) | 2.9 (1.2–5.3) | 0.01 | ||
| Charlson index | 2.7 (2) | 3.1 (2.5) | 2.4 (1.8) | 1.5 (0.67–3.5) | n.s. (0.18) | ||
| Presence of COPD (n) | 61 (61) | 21 (63) | 40 (60) | 1.5 (0.85–2.6) | n.s. (0.24) | ||
| Pneumonia (n) | 10 (10) | 4 (12) | 6 (9) | 1.1 (0.35–3.3) | n.s. (0.9) | ||
| Septic shock (n) | 19 (19) | 17 (52) | 2 (3) | 14 (4–37) | <0.0001 | 7.2 (2–19) | <0.001 |
| ARDS (n) | 7 (7) | 2 (6) | 5 (7.5) | 0.7 (0.55–2.7) | n.s. (0.7) | ||
| ΔPL (cmH2O) | 23.7 (14.2) | 24 (12.4) | 23.6 (15.1) | 0.85 (0.67–4.9) | n.s. (0.85) | ||
| ΔPaw (cmH2O) | 9 (6.5) | 10.7 (7.7) | 8 (4.9) | 1.6 (0.67–2.6) | n.s. (0.7) | ||
| TF < 20% | 17 (17) | 13 (39.4) | 4 (6) | 3.17 (1.9–4.9) | <0.0001 | 3.7 (1.1–6.9) | 0.003 |
| C reactive protein (mg/dl) | 14 (13.4) | 9.7 (8.5) | 18.5 (14.6) | 0.4 (0.1–1.7) | n.s. (0.08) | ||
| Procalcitonin (ng/L) | 8.6 (5.5) | 14.4 (4.2) | 6.9 (6.2) | 1.8 (0.67–5.9) | n.s. (0.25) | ||
| Glucose (mg/dL) | 155 (45) | 156 (37) | 154.7 (49) | 1 (0.80–1.3) | n.s. (0.9) | ||
| Albumin (mg/dL) | 3 (1.7) | 2.9 (1.5) | 3.1 (1.9) | 1.7 (0.78–2.56) | n.s. (0.15) | ||
| Creatinine (mg/l) | 1.29 (0.8) | 1.3 (0.76) | 1.27 (0.9) | 1.1 (0.77–1.9) | n.s. (0.44) | ||
| Lactate (mg/dL) | 17 (10) | 22.34 (12) | 14.2 (9) | 3.5 (0.8–9.2) | n.s. (0.07) | ||
| pH | 7.33 (0.1) | 7.29 (0.12) | 7.36 (0.1) | 2.9 (0.91–6.7) | n.s. (0.06) | ||
| P/F ratio | 189 (55) | 206 (61) | 165 (52) | 1.4 (0.7–2-3) | n.s. (0.32) | ||
| Arterial pCO2 | 60 (11) | 66.6 (14) | 56 (9) | 3.4 (0.81–6.7) | n.s. (0.09) | ||
| Recent hospitalizationa (n) | 29 (29) | 15 (45) | 14 (21) | 1.8 (1–3.6) | n.s. (0.06) | ||
| Multidrug resistant (MDR) micro-organism colonization | 26 (26) | 13 (39) | 13 (20) | 1.7 (0.9–3.2) | n.s. (0.14) | ||
| MDR infection | 12 (12) | 9 (27) | 3 (4.5) | 4.7 (1.6–13) | 0.009 | 4.7 (1.3–9) | 0.021 |
| Second infection during RICU stay | 33 (33) | 17 (52) | 16 (24) | 3.17 (1.1–3.1) | 0.031 | 1.9 (1.1–4.5) | 0.027 |
| CRP worsening (n) | 33 (33) | 17 (52) | 16 (24) | 3.1 (2–8.1) | 0.006 | 2.1 (1.4–6.1) | 0.036 |
Data are presented as number and percentage for dichotomous values or mean value and standard deviation for continuous values.
Table 1 also shows the results from the univariate and the multivariate analysis performed to identify potential predictors of chronic CI development in the study cohort.
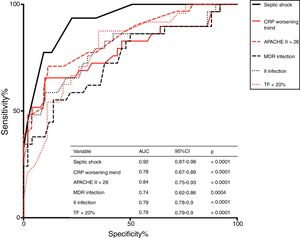
Higher APACHE II score on admission, the presence of septic shock, DD, and MDR infection, the onset of a second infection during stay, and a trend of CRP to increase at day 7 after admission were independently associated with chronic CI development; ROC analysis indicated that septic shock was the strongest predictor (AUC = 0.92, p < 0.0001) of chronic CI onset (see Fig. 3).
DiscussionWith this cohort study, we have observed a 33% prevalence of chronic CI in patients admitted to RICU due to de novo ARF. We have also shown that a worse clinical severity and diaphragmatic dysfunction at admission, early worsening of systemic inflammation, type of infection, and septic shock in particular, are factors independently associated with chronic CI development.
Prevalence of chronic CI in the ICUGiven the lack of a univocal definition of chronic CI, the exact prevalence of this condition remains unknown. As a whole in patients admitted to ICUs, the prevalence of chronic CI defined as the need to prolong MV is around 10%.1 Moreover, chronic CI represents a predominant clinical feature in up to 50% of patients who survive an episode of sepsis.10 In our study performed in a medical specialized ICU in patients with de novo ARF, onset of chronic CI was lower than that reported in a surgical ICU.10 However, in the latter study10 the definition of chronic CI referred to the length of stay in ICU for ≥14 days with persistent organ dysfunction as assessed by SOFA score. In our study, chronic CI was defined as the need for respiratory intensive care for ≥8 days associated with the decision to perform tracheostomy and to prolong MV. Prolonged mechanical ventilation and tracheostomy are factors that increase per se the risk of bed-lying, thus requiring combined strategies to avoid complications and speed-up recovery in specialized intensive care settings.22
Inflammation and chronic CIDuring stay in ICU, persistence of inflammatory state is the pathophysiological key which may favor chronic CI development.23 The term “persistent inflammation, immunosuppression and catabolism syndrome” (PICS) has been coined to describe a phenotype of chronic CI in surgical ICU patients who suffered from major pro-inflammatory event (blunt traumatic injury/sepsis).24,25 A self-perpetuating cycle of inflammation constitutes the pathobiological hypothesis underlying PICS development.26 Patients who develop chronic CI after sepsis exhibit persistent elevations of systemic inflammatory cytokines (IL-6 and IL-8) even at 28 days following onset of ARF,21 which is the case of the inflammatory response during ARDS.27 Persistence of elevated levels of inflammatory cytokines and chemokines in circulating fluids and bronchoalveolar lavage, could lead patients with ARF to chronic CI.28 In patients with ARDS, longitudinal sampling has proved that local and systemic inflammation may persist for several weeks, even after the resolution of the respiratory syndrome.29 Recently, both hyperinflammatory and hypoinflammatory subphenotypes were identified analyzing biomarkers plasma levels from two trials in patients with ARDS.30 The hyperinflammatory subphenotype is characterized by fewer days free from ventilatory and/or organ failure, conditions which constitute a risk factor for chronic CI. In our study, the measurement during the first week after admission of CRP as a marker of inflammation was able to distinguish the group of patients who would develop chronic CI from those who did not and would recover (Fig. 2).
Infection and chronic CITo study the underlying mechanisms that drive chronic CI progression over the course of sepsis, different animal models and clinical studies in survivors have been conducted.30–32 Data show that immunological dysfunction and repeated infections have a role in maintaining inflammation and, finally, trigger chronic CI.33,34 In line with these findings, our study confirms that patients with ARF presenting a second infection during stay in RICU or having a MDR bacterial infection are more likely to develop chronic CI. Overall, this suggests that immunological dysregulation and susceptibility to infection constitute a mechanism favoring the development of chronic CI in these individuals. Interestingly, septic shock, a condition characterized by profound circulatory, cellular and metabolic alterations, was the strongest predictor of chronic CI development in our study (Fig. 3).
DD and chronic CIIn patients suffering from acute critical illness of various etiologies, respiratory muscle dysfunction is quite frequent and may develop early. Demoule et al. have shown that 64% of patients admitted to ICU have DD at the acute onset, sepsis being the major independent risk factor.35 The mechanisms underlying sepsis-induced DD (SIDD) are complex.36 At the molecular level, there is evidence that cytokines (TNF-alpha, IL-1alpha, IL-1beta and IL-6) and inflammatory signaling (NF-kappaB pathways) are involved,37 and may promote mitochondrial dysfunction, reactive oxygen species (ROS) production, and breakdown of sarcomere proteins during infection.38 In the present study, we have reported that DD (i.e. TF < 20%) by noninvasive assessment early at the time of admission is a risk factor in the specific population of difficult-to-wean patients. To the best of our knowledge, this result is new and opens further research perspectives.
Ventilatory induced lung injury and chronic CIThere is evidence in literature that indicates that MV plays a role in determining ventilator-induced lung injury.39 In particular, non-physiological stress/strain could act as a trigger and amplifier for local and systemic inflammation.40 In our study, we measured ΔPaw as a surrogate marker for cyclic lung strain in patients receiving ventilator support41 and ΔPL as a marker of lung stress in patients with ASB16; however, we failed to find any correlation with chronic CI development. Therefore, even though lung mechanics have been used with a small group of patients, data suggest that mechanical stress/strain on lung parenchyma might play only a minor role in the physiopathology behind the onset of chronic CI.
LimitationsDespite intriguing findings our observational study presents some major problems which limit generalizability. First, the evidence of persistent inflammation as a potential risk for chronic CI development lacks measurement of specific inflammatory cytokines. Second, immunological assessment has not been investigated in our population. Third, the definition of chronic CI as applied here excludes those patients with persistent signs of organ dysfunction (i.e. renal insufficiency), or without any need for tracheostomy and prolonged MV.
ConclusionsPatients admitted to RICU with de novo ARF frequently progress to chronic CI, infection-related factors being crucial in the pathogenesis. Data here presented warrant further research to clarify all the potential physio-pathological factors predisposing patients to chronic CI development.
Ethics approval and consent to participateApproval from the local ethics committee of Modena was obtained (registered protocol number protocol 839/C.E. and 266/16 C.E). Written informed consent to participate was obtained by all patients enrolled.
Consent for publicationConsent for publication was obtained by all patients enrolled.
Availability of data and materialsData are available at the Respiratory Disease Unit of the University Hospital of Modena, Italy.
Declarations of interestsNone.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to thank Colin Woodham at Alpha Science Editors for language editing.