In order to measure cardiopulmonary performance for clinical and investigation purposes we need standardized tests which allow the comparison with standard values, between people, or individuals with themselves over time. The quest for the ideal exercise test has led to the development of several formats, the so called laboratory and field tests. Incremental exercise tests allow measurement of maximal exercise capacity and a host of submaximal variables of great interest. The physiological rationale of the tests and of the detection of interesting submaximal variables can be explained from the oxygen uptake and carbon dioxide output kinetic response to constant power exercise. When the muscles have to produce very high energy, the exercise is physiologically limited to relatively short duration. The minimum power at which an exercise can no longer be sustained for long periods of time is called critical power. Above critical power the time-power function shows a hyperbolic shape. This shape provides the rationale for understanding the properties, limitations and responsiveness to interventions of endurance tests such as constant power test on a cycle-ergometer or treadmill, endurance shuttle walk test and six-minute walk test.
Performance during standardized exercise tests (i.e. laboratory and field tests) and their associated physiological or pathophysiological responses are recognized biomarkers of considerable importance in the multidimensional evaluation of cardiac and respiratory diseases.1–4 From an evidence-based perspective, exercise testing is fundamental to accurately quantify cardiorespiratory fitness,1–4 it may uncover the pathophysiologic mechanisms underlying exercise intolerance and it is independently related to major outcomes such as survival and hospital admissions.1–5 Therefore, exercise capacity assessment is a valuable tool to evaluate the severity of impairment, as it provides meaningful clues for tailoring individualized rehabilitative interventions and considerably improves prognostic stratification.
In order to measure cardiopulmonary performance for clinical and investigation purposes we need standardized tests which enable comparison with standard values, between different people, or individuals with themselves over time. These tests are broadly divided into laboratory-based exercise tests and field tests. Laboratory tests are usually conducted on either a cycle-ergometer or a motorized treadmill. A comprehensive array of physiological system response can be measured, which provide for accurate definition of responses both at the limit of tolerance and throughout the course of the test. On the other hand we have the so called field tests that consist of asking the persons being tested to walk at a specific rate or the fastest pace possible during a specific time. In these tests the physiological information attainable is usually limited.
In this review we will start by describing the responses of the oxygen and carbon dioxide transport and utilization/production systems to a single step increase of power, since they are the cornerstone to understanding the physiological rationale and limitations of all exercise tests.
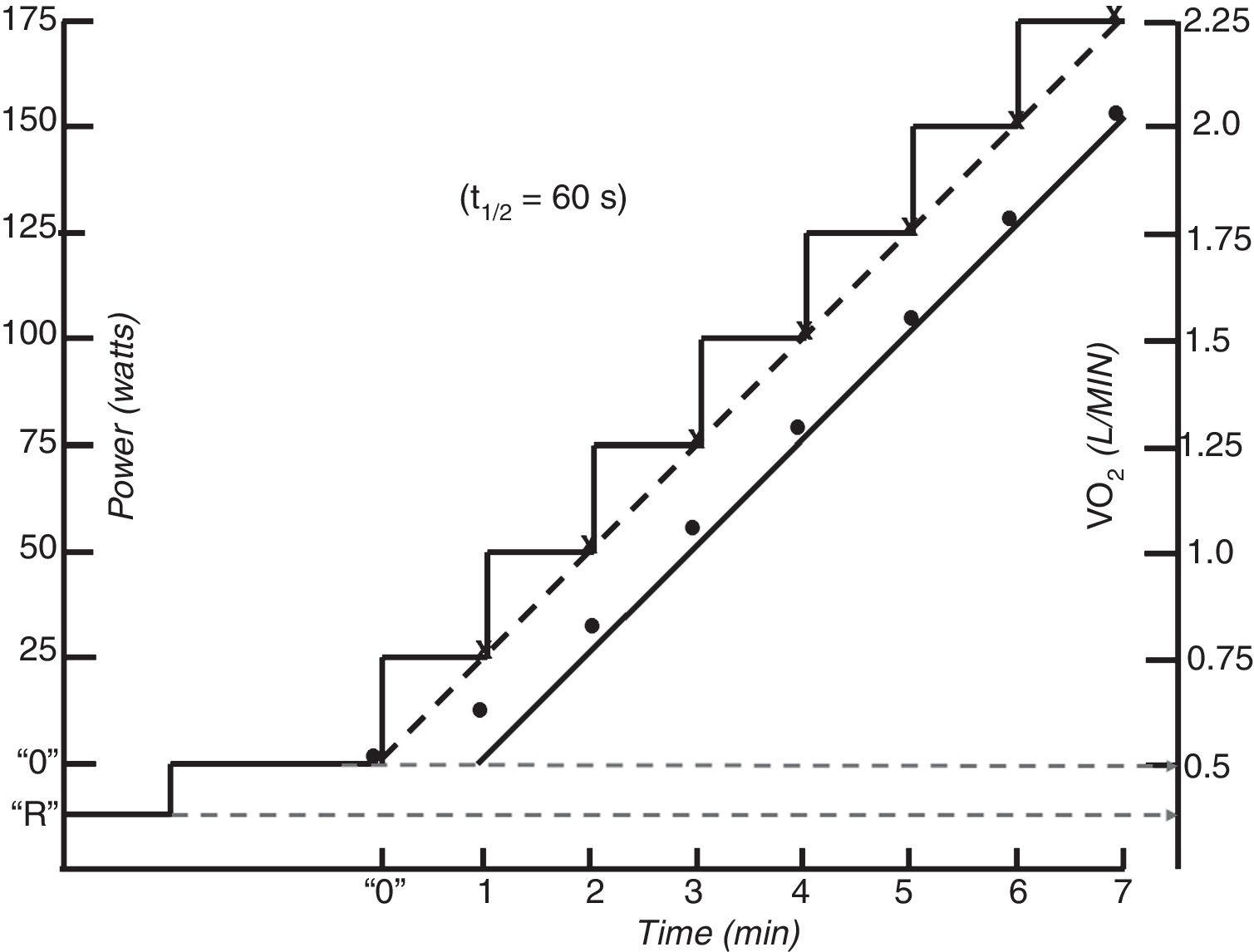
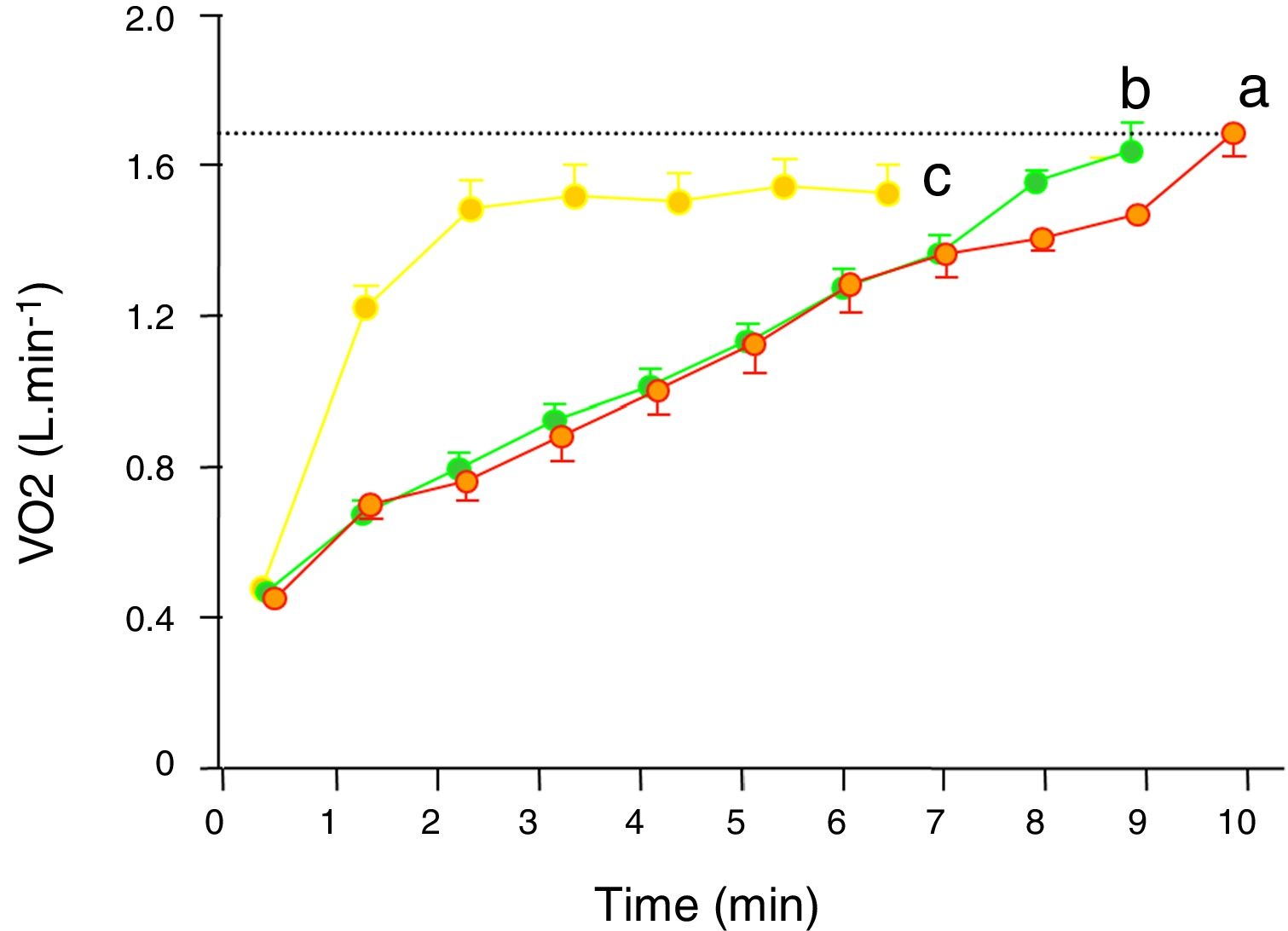
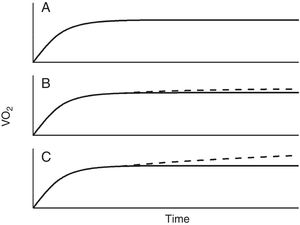
Single-step moderate constant power exerciseIn repose to the onset of constant exercise in the upright position, oxygen uptake (V’O2) initially rises abruptly (phase 1 component). Phase 1 is the gas-exchange expression of the immediate increase in venous blood flow through the lungs as a consequence of both the compression of the veins by contracting muscles and the activation of the sympathetic nervous system leading to increased cardiac chrono- and inotropy and to venous vasoconstriction.5 Subsequently a more dominant exponential phase 2 component develops, determined by the hemodynamic response (increase in cardiac output and venous extraction in the working muscles). In moderate intensity of exercise (i.e. exercise at which no sustained increase in blood lactate takes place) phase 2I response follows a single-exponential growing function. As phase I is very difficult to identify in single breath-by-breath traces, for the interest of this review, the initial increase in VO2 can also be modelled by a single exponential growing curve encompassing phase 1 and 2, which time constant is called mean response time (MRT) and is typically between of 30–45 sec in healthy people.5–8 MRT is the amount of time that it takes to increase VO by a factor of 1-1/e (because 1/e is approximately 0.368, MRT is the amount of time that takes VO2 to increase to approximately 63.2% of what it is needed to reach the steady state situation (i.e. oxygen supply and demand are matched) corresponding to a given moderate power. Eventually (after a time span of 4× mean response time ˜ 2 −3 min) such a steady state (phase 3 component) is achieved (Fig. 1).5,9 The steady state VO2 attained in phase 3 s at any exercise intensity of the moderate domain keeps a remarkably constant relationship with power (VO2 /Δ power) is ˜10 ml · min−1·W−1 with slight variations depending on the relative proportions of carbohydrate and fatty acids being catabolized and the involved muscle-fibers.5
Carbon dioxide output (V’CO2) follows the same pattern of response, but because at the beginning of exercise the body is able to accumulate a fair amount of the carbon dioxide produced by the working muscles MRT for VCO2 is longer (50–60 s) and it usually takes V’CO2 around 4 min to reach its steady state 5–7. Ventilation (V’E) follows V’CO2 with a slight delay5–7,10 and heart rate is faster than V’O25–8,11,12 (Fig. 1).
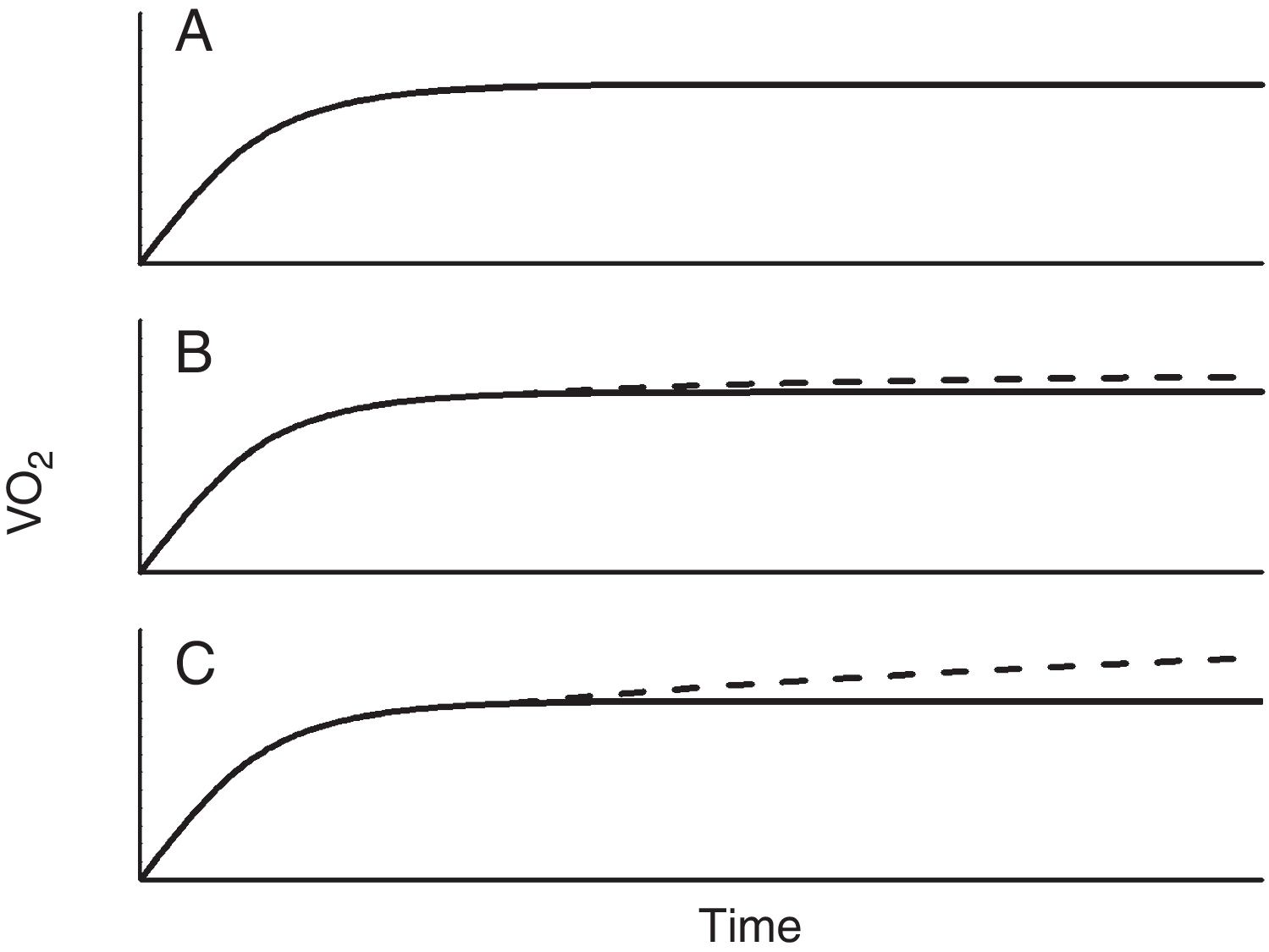
Single-step intense constant power exerciseIn contrast to moderate-intensity exercise, which duration will depend on factors such as availability of fuels (mainly glucose, but also certain proportion of fatty acids (especially in trained subjects), dehydration or in real life locomotor or feet injuries, intense exercise is limited by factors related with the oxygen flow to the muscles and deteriorating homeostasis when it does not match the demands. It is beyond the scope of this work to discuss whether there is a bioenergetics threshold at the cellular level, however what it is clear and has been repeatedly demonstrated, is that above a certain intensity exercise, V’O2 kinetics becomes unquestionably different from moderate exercise (Fig. 2).13–15 Thus, above certain repeatable threshold, the VO2 keeps on rising after the third minute, resulting in a greater VO2 proportion with respect to the power than with moderate exercise.5,13–15 While the physiology of this extra VO2 (usually called the second component) is not completely understood, it has been modelled as an additional slow and delayed (by several minutes) VO2 component superimposed on the phase 2 response.5 The region of intense exercise can be subdivided in two domains regarding lactic acid accumulation and VO2 rise (Fig. 2). Below certain exercise intensity, called critical power (CP),16,17 the increase peripheral oxygen extraction by ensuing acidemia and the metabolic transformation of lactate back to pyruvate by the liver and less active muscles18,19 can match the lactic acid produced by the exercising muscles and an equilibrium is achieved, discernible because VO2 and lactic acid rising ends and a new steady state is reached.5,13–15 Above the CP, though, a steady state is not achievable and VO2 rises either to its maximum, presumable because the upper limit for O2 conductance and utilization is achieved,1,5,15 or to the tolerable limit, because the symptoms associated with approaching to the physiological limits compel the individual to quit.1,5
It is not clear whether the “second component” of V’O2 derives into increased V’CO2,7 nonetheless, in the domain of intense exercise new sources of carbon dioxide are added to the metabolic V’CO2. In first place the additional carbon dioxide released by the bicarbonate buffering of lactic acid becomes noticeable soon after lactic acidosis develops and muscle and blood bicarbonate are utilized.5 V’CO2 consequently becomes higher than V’O2 (since working muscles use mainly glucose as fuel, their respiratory quotient is close to 1 and so does the respiratory exchange ratio (RER) when the muscles are the main metabolic source of carbon dioxide—as it happens in exercise— therefore RER becomes higher than 1 when bicarbonate buffering of lactic acid takes place. As bicarbonate is consumed, this source of carbon dioxide fades away and a different source of carbon dioxide takes over i.e. the carbon dioxide washed out from the body to offset the ongoing lactic acidemia, what it is manifested as hypocapnia.5,6,10,14,20 The intense exercise V’CO2 kinetic response frequently appears as monoexponential, but this resemblance is deceptive since it is the result of the coincidence of several physiological mechanisms with different temporal characteristics.7,21
As with moderate intensity exercise, VE follows V’CO2 until acidosis develops, afterwards it is driven by the falling pH and hyperventilation, and it is VE that starts driving the extra V’CO2 release from body carbon dioxide stores (hyperventilation).5,6,10,14,20 In heavy exercise as in moderate exercise heart rate kinetics is faster than V’O2, nonetheless, heart rate does not reach a steady state, rather it keeps increasing parallel to V’O2.5,19
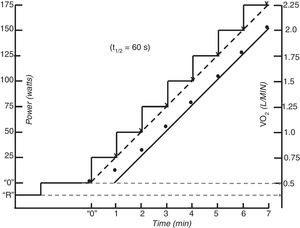
Laboratory testsIncremental testsIncremental exercise tests are tests in which the power increase is a linear function of time. These tests are aimed at maximally stressing the O’2 transport and utilization systems. They are routinely used in the clinical setting because they measure maximal aerobic capacity and provide information about all the physiological domains of exercise (i.e. moderate, heavy and very heavy) as well, in a compact format. These tests consist of either 1-min increases in power or the so called ramp tests with continuous (every 2–5 sec) escalations of power. Imposing linear increase profiles for treadmill exercise can be problematic because most speed/grade increments incorporated into clinical exercise testing do not result in linear increases in power.22
The cycle-ergometer is used more often than the treadmill because it is less expensive, occupies little space, is less prone to movement artefacts, makes it easier to take additional measurements, requires relatively little practice and unlike the treadmill, the external power output is accurately known.1,4,5,23 On the other hand, walking on treadmill is more familiar for the patient, and has been proposed that it may better reflect an exercise modality encountered during daily living.1,4,5,23 Physiological responses to cycle ergometer and treadmill tests differ and so can the physiological factors limiting exercise tolerance. For example, in patients with pulmonary disease, cycle-ergometry results in a greater likelihood for exercise intolerance to result from leg fatigue rather than dyspnoea.1,4,5,23–26 However, desaturation occurs more frequently walking than cycling in respiratory patients.27
The normal V’O2 response to incremental test is the direct consequence of the response to moderate constant power exercise. The expected V’O2 increase approximately 10 ml · min−1·W−1 (see before).5 As the incremental test can be considered to be the continuous sequential summation of constant power stimulus, then the expected V’O2 accumulated response will be also the sequential summation of each step response. Hence, the linear phase of V’O2 lags behind the steady state V’O2 response by the MRT.8,28 (Fig. 1). Because steady states are never achieved in incremental protocols,29 the actual V’O2 at any instant of the moderate intensity region will always be inferior to what it is expected for the isochronal power (i.e. ˜10 ml · min−1·W−1 plus unload pedaling V’O2. This linearity is no longer possible at powers over the LAT due to the superimposition of the V’O2 “slow component” to the anticipated value.5,13–15 It would be expected that above latacte thershold the slope of V’O2 would become higher, however as the slow component is very sluggish, it has been empirically shown that it becomes less than expected at very rapid power increments for the fitness of the person (due to a high anaerobic metabolism), about the same for intermediate power rises and greater, as expected, for slow power increments.30 Thus, while the speed of the test does not usually affect the peak V’O2 it changes the V’O2 - WR relationship, making peak power a poor surrogate of peak V’O2.
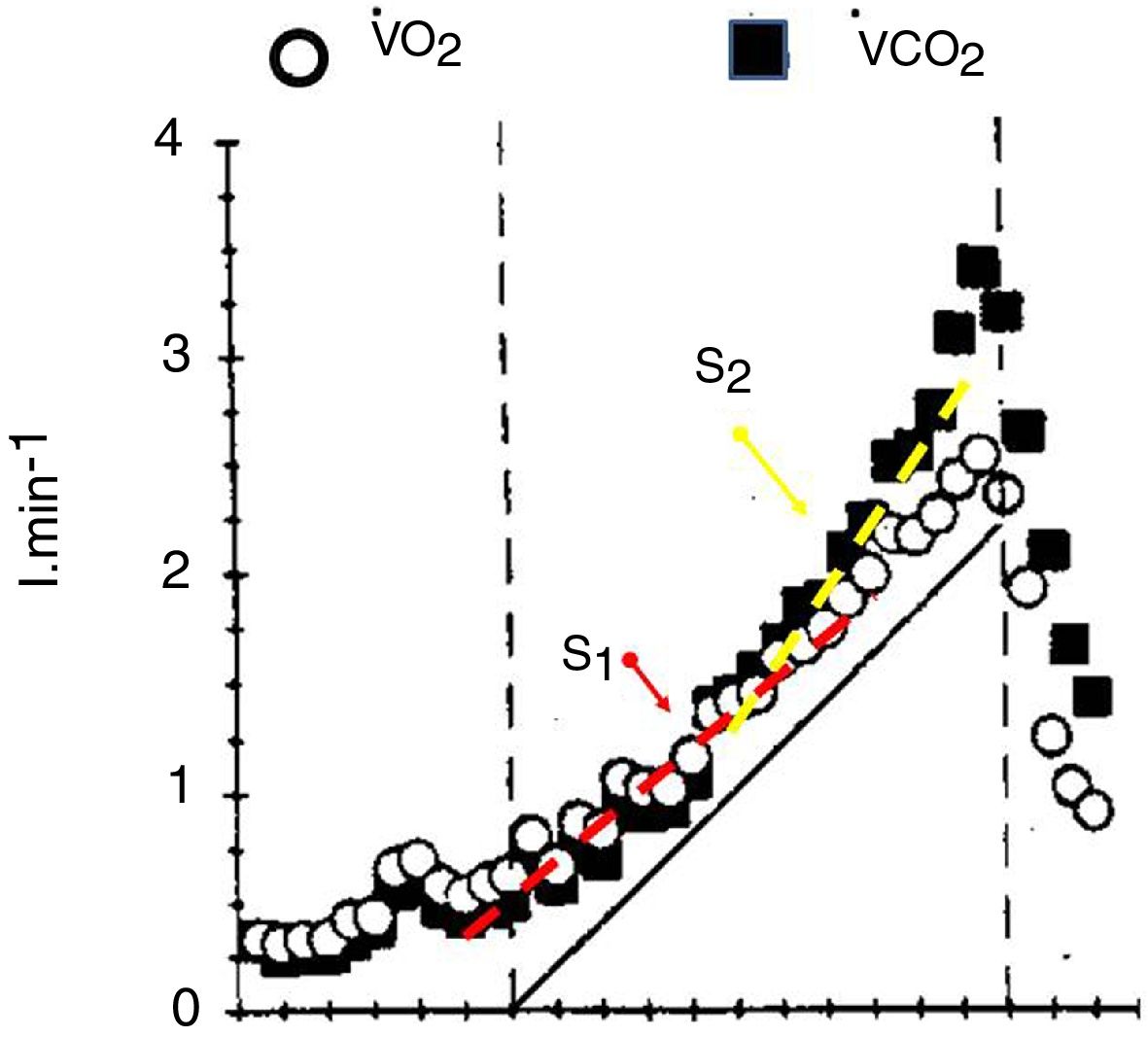
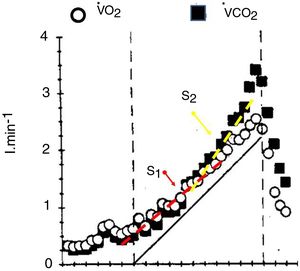
The V’CO2 response to incremental exercise tests relative to V’O2 in the sub-LAT region is displaced further from the VCO2 steady state to power relationship because of its slower kinetics.5,7 This is the reflection of the part of the metabolic carbon dioxide retained in the body’s stores.5,7 Thus, in this domain of exercise, following an initial period of transient carbon dioxide stores accumulation, which may last up to 3 min, the V’CO2 ramp response also becomes relatively linear (S1 slope) with respect to power. For this reason, in the moderate exercise region RER at the lung will slightly underestimate the tissue respiratory quotient.31
At powers above the lactate threshold, the addition of carbon dioxide coming from bicarbonate buffering of lactic acid, drives V’CO2 to an steeper slope with respect to the power increase, this region has been called isocapnic buffering region or “S2”7 (Fig. 3), This change in slope is the physiological rationale for the non-invasive detection of the lactate threshold.5,7 When the blood bicarbonate becomes insufficient to buffer the lactic acid produced, a new source of carbon dioxide takes over i.e. the hyperventilation phase, characterized by the decrement in end-tidal arterial carbon dioxide pressure and a third change in slope (Fig. 3). The point at which this second change in slope is discernible is called the respiratory compensation point.
The faster the increases in power, the greater the lactic acid production, and therefore the greater the S2 slope and therefore the RER at maximum exercise may increase at levels higher than 1.2 with rapid tests, but only 1.05 in slower test.5,7,31 This limits the usefulness of RER as marker of good effort and underlines one of the drawbacks of this format, the standardization of the speed of the power increments, particularly in sick individuals. It is beyond the scope of this review to describe the proposed methods to standardize the speed of the test. The reader is referred to further texts.4,5,32
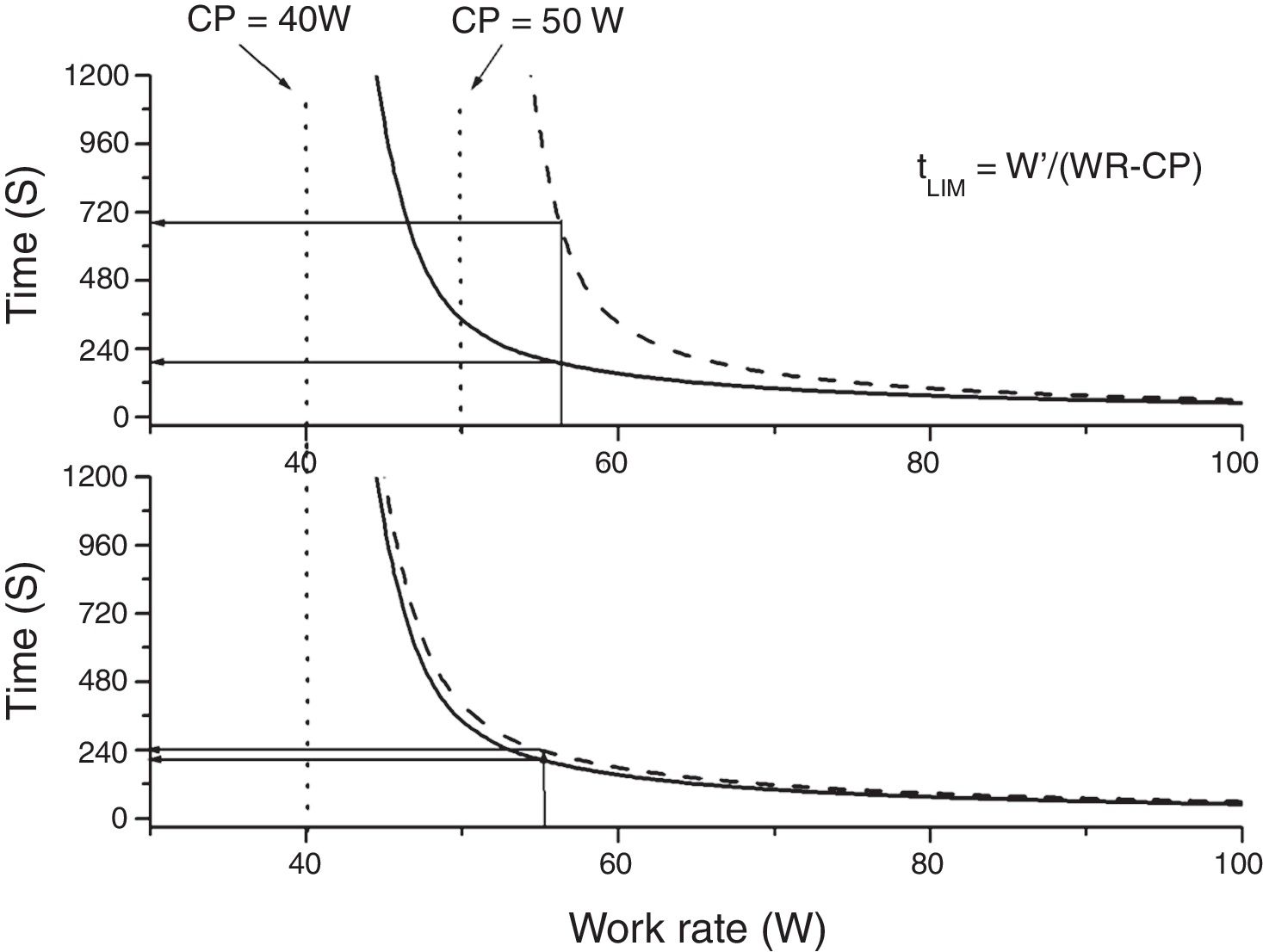
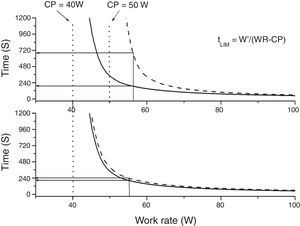
Constant power (endurance) testsThe relationship between power and endurance time (tLIM) for constant-work-rate exercise is hyperbolic 33,34, (Fig. 4):
where P is the imposed power and W' is the curvature constant (having units of work). CP, as we said before, represents the highest power that can be maintained without VO2 continuing to increase with time towards VO2max rather than attaining a submaximal steady state.From the hyperbolic shape of the power-duration relationship (Fig. 4), it can be inferred that both the duration of the test and the magnitude of its response to an intervention will depend on the position of the selected power on the power-duration relationship.35 The practical expedient of selecting a fixed percentage of the peak power obtained on a prior incremental exercise test takes no account of such issues, since CP does not occur at a fixed percentage of the peak V’O2 (or peak power). This has two major consequences: firstly, the inter-subject variability will be greater than if the intensity for the constant test were normalized to CP35,36 and secondly, for interventions that increase CP, improvements in tLIM are highly dependent on the difference between selected power for the endurance test and CP.35,36 Thus, the use of tLIM as a robust measure of efficacy of interventions—as well as in other non-time-limited endurance tests such as the endurance shuttle walking test (ESWT)—for which improvement depends on the magnitude of effect of interventions on CP - requires that the pre-intervention intensity be normalized for the fitness (i.e. CP) of the tested person. However, estimating CP is cumbersome, requiring the performance of several constant tests above the LAT. A practical approach to normalizing constant power test intensity when CP is not known has been proposed.4
Field testsThe incremental shuttle walking testThe incremental shuttle walking test (ISWT) is an externally-paced incremental walking test.37 Subjects are required to walk around two markers 9 m apart (10 m course). Single audio cues (beeps) signal the time at which the subject is expected to turn at the marker. Walking speed is increased each minute. The ISWT has 12 levels (walking speeds) and therefore lasts a maximum of 12 min. No encouragements are given during the test: the only verbal cues provided refer to an impending increase in walking speed.37 While the profile of the test is not exactly linear, it is very close38 (Fig. 5) and available data suggests that ISWT distance correlates well (r = 0.66-0.88) with measured peak V’O2 in incremental tests.4,39 ISWT performance is usually defined as the distance achieved.
Endurance shuttle walking testThe ESWT40 is derived from the ISWT, much like the laboratory constant-power test derives from the incremental tests. Like the ISWT, it is externally paced and its intensity is tailored to the exercise tolerance of the individual patient. The ESWT uses the same course and auditory signal method as the ISWT, however, a constant walking cadence is maintained throughout the test. The ESWT starts with a 100 s “warm-up” at a slow pace,40 followed by the “exercise” phase at the prescribed speed (typically 80% of ISWT peak) calculated from a previous ISWT.40 Results are expressed in seconds or in metres.
As the considerations with respect to the power-duration curve for the ESWT are essentially identical to those of the constant-work-rate tests, inter-individual variability of test duration is expected to be high, unless the duration is purposely standardized. There is little information on the mechanisms determining baseline ESWT duration. It is likely that the physiological determinants are analogous to those of constant power test although some evidence suggests that ventilatory limitation may be more prominent in walking than in cycle-ergometry.41 It is possible that some patients may fail because of a ceiling (see below) effect.42
Six-minute walking testDuring the six-minute walk test the patient is encouraged to walk at the maximum possible speed compatible with covering as much distance as possible in 6 min. Individuals acquainted with the test and able to complete it in one bout, tend to select a roughly constant pace throughout the test38 (Fig. 5). Therefore, although the test is not intended to be a constant test, the power (i.e. the speed at which patients carry their own weight during walking) is usually fairly constant,38 as illustrated by the attainment of a plateau in the oxygen uptake and heart rate responses during the test.38,43 Moreover, it has been reported that, in individuals familiar with the test and with encouragement, the selected walking speed is comparable to the critical speed (the equivalent of critical power for walking).38,43 Thus six-minute walk test comes to be a kind of constant power test38,43 (Fig. 5). However, two particular features make it different, and potentially less sensitive, to interventions than proper endurance tests.43 On the one hand several studies have shown that respiratory patients tend to select a relatively constant speed of walking38,43 and, unless walking itself is specifically trained,45 they may not adopt a faster walking pace after interventions improving their pulmonary function.43,44 On the other hand six-minute walk has been shown to have a ceiling effect, as the linear relationship between peak VO2 and six-minute walk test is lost in less impaired subjects.43,46
ConclusionsPerformance during standardized exercise tests (i.e. laboratory and field tests) and their associated physiological or pathophysiological responses are recognized biomarkers of considerable importance in the multidimensional evaluation of cardiac and respiratory diseases.
The oxygen uptake (V’O2) response when the skeletal muscles have to generate a moderate intensity constant power can be described by a monoexponetial function with an amplitude of 10 ml · min−1·W−1 and a MRT usually between 30 and 60 s. At this steady state the oxygen supply matches the oxygen demand of the working muscles. For exercise of higher intensity—coincident with the accumulation of lactate—the V’O2 continues to rise above the steady-state amplitude reached at moderate exercise until a new steady state is attained—this time because lactate metabolism by the liver and less active muscles are able to metabolize the lactate produce by the working muscles—or, at higher intensities until the maximum VO2 is reached or closely approached. The minimum power at which this second steady state is no longer possible is called critical power (CP). The time that exercises above CP can be sustained is limited and can be modelled as a hyperbolic function of power. From these physiological concepts, on the one hand the amplitude and MRT and on the other the hyperbolic shape of the power- duration relationship, can be explained the profile of VO2 in incremental test and the duration and responsiveness to interventions of constant work rate tests respectively.
VCO2 profile is also exponential, but slower because part of the carbon dioxide produced by the active muscles is dissolved in the body water, however its kinetic response is the result of the coincidence of several physiological mechanisms with different temporal characteristics. Indeed VCO2 comes mainly from three different sources: metabolism, buffering of lactate by bicarbonate and hyperventilation. The different kinetics of these three sources provides the rationale for the detection of the LAT and for the standardization of the speed of the test.