Pulmonary veno-occlusive disease (PVOD) and pulmonary capillary hemangiomatosis (PCH) have been considered separate entities for a long time. However, there has been increasing evidence that these lesions may represent the extremes of the same process that involves the venules, the interlobular veins with partial or complete fibrous obstruction of their lumen and the alveolar pulmonary capillaries showing dilatation/hyperplasia.1
They are included in group 1.5 of the sixth World Classification.2 However, when one of the two extremes prevails (PCH vs VOD), the Computed Tomography (CT) scan aspects diverge. Ground glass centrilobular nodules are more evident in PCH, while thickening of interlobular septa and bilateral pleural effusion are the hallmarks of CT in VOD.3,4
Prognosis is very poor and there is no established medical treatment, although quite recently imatinib has been shown to improve pulmonary hemodynamics.5 Here a case of PCH diagnosed by transbronchial lung cryobiopsy (TBLC) is described. A 31-year-old man came to our attention for dyspnea on effort. He was an immigrant from Senegal, in Italy for12 years, an active smoker (with a 10 pack-year history) and had been working in a chicken-processing factory for the last 5 years. He had a history of mild asthma, and his family history included the death of a younger brother due to unknown respiratory problems. The physical examination was not relevant. Oxygen saturation at rest while breathing room air was 94 %. Arterial blood gas analysis (on room air) documented only hypocapnia, the six minute walking test revealed a significant functional limitation (125 mts; O2 saturation up to 83 %). Pulmonary function tests showed a FVC 82 % of predicted, FEV1 72 % of predicted, consistent with a mild obstruction and a severe reduction in diffusion capacity for carbon monoxide (DLCO) (16 % of predicted). Blood tests showed high levels of precipitins for avian antigens (pigeons, parakeets). An extended autoimmunity panel, including myositis antibodies, returned negative, as did serology for hepatitis viruses, HIV and QuantiFERON test. Reactive C protein and white blood cells counts were within normal range.
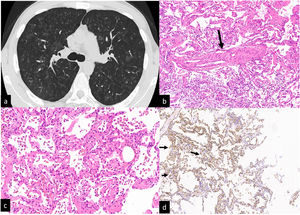
A pulmonary CT pulmonary angiogram ruled out pulmonary embolism, documented the presence of multiple, diffuse, centrilobular ground-glass nodules, a few intraparenchymal cysts, and an increased caliber of the main trunk of the pulmonary artery (Fig. 1 a). Areas of air trapping were not present even in the expiratory phase. A hypothesis of PCH was suggested by our thoracic Radiologist with hypersensitivity pneumonitis with pulmonary hypertension as a second, less probable, hypothesis.
CT scan without contrast. Multiple ill-defined ground glass centrilobular nodules distributed to both lungs. No mosaic attenuation related to air trapping is visible (a). Transbronchial cryiobiopsy:an interlobular vein with the lumen partially obstructed by fibrous tissue (b, arrow). Interstitial thickening is due to hyperplastic/dilated capillary-like vessels (arranged in superimposed rows). Hemosiderin-laden macrophages in the alveolar spaces (c). Alveolar capillaries marked by CD31 monoclonal antibodies are arranged in superimposed rows (d, arrows).
Transthoracic echocardiogram showed a pulmonary artery hypertension (sPAP 52 mmHg) later confirmed by right heart catheterization as pre-capillary (mean pulmonary arterial pression 34 mmHg, pulmonary capillary wedge pressure 10 mmHg, pulmonary vascular resistance 4.25WU). The multidisciplinary discussion took into consideration the two following hypothesis: PCH and hypersensitivity pneumonitis. A bronchoalveolar lavage with instillation of 150 mL of saline showed a normal cytological profile and microbiologic tests were negative. Eventually a transbronchial lung biopsy (TBLC) was requested. It was carried out using the method already described.6 Four samples were retrieved: one in the lateral segment of the lower right lobe, one in the meddle lobe and two in the posterior segment of the right upper lobe.
After the procedure, the patient developed a pneumothorax resolved in two days after the positioning of a chest tube. The four samples had a total area (measured under the microscope) of 189.11 mm2. Visceral pleura was present only in one sample. Histopathologic findings were: pulmonary veins in the interlobular septa with a lumen partly obliterated by fibrous tissue; vaguely nodular areas showing thickening of the interalveolar septa by the presence of dilated capillary-like vessels superimposed in more than one row. The surrounding alveolar spaces contained hemosiderin laden macrophages. The endothelial cells of the dilated capillaries were marked by anti CD31 monoclonal antibodies (Fig 1b-2d). A diagnosis of PCH/VOD was later confirmed. An off-label treatment with Imatinib was started and the patient was referred for lung transplantation.
TBLC is now suggested as a valid alternative to surgical lung biopsy for the diagnosis of diffuse parenchymal lung disorders with a diagnostic yield around 80 % and an acceptable rate of complications.7-9 However, the presence of significant lung impairment including reduction of DLCO<35 % of predicted and of pulmonary hypertension (sPAP by echocardiography ≥45 mmHg) are considered a relative contraindication in the statements and guidelines published so far,7–9 mainly because pulmonary hypertension could represent a risk factor for major, life threatening, bleeding. Adverse events associated to TCLB in subjects with significant function lung impairment and/or pulmonary hypertension were reported by Bondue et al.10 The cohort included 38 patients with undiagnosed ILD at high risk for SLB (defined as age ≥75-years, body mass index ≥35, sPAP by echocardiography ≥45 mmHg, forced vital capacity <50 %, diffusing capacity for carbon monoxide <30 %, and/or significant cardiac comorbidities with reduced heart ejection fraction) and it was compared with 58 patients at low risk.10 Numbers of bleeding, pneumothorax, mortality and hospital stay were almost identical in both groups. Two patients with pulmonary hypertension submitted to transbronchial cryobiopsy with a final diagnosis of intravascular large B cell lymphoma and no complications were reported by Poletti V et al.11 In the case here reported there were two elements for considering lung biopsy a risky procedure (elevated PAP and a very low DLCO). However, the documented exposure to animal proteins, the presence of serum precipitins against avium antigens, the presence of centrilobular nodules with a few cysts in the CT scan, did plausible also the hypothesis of hypersensitivity pneumonitis. TBLC was crucial for the final diagnosis. The size of TBLC samples was reported to be a key factor for the diagnostic yield of TLCB by Casoni G et al.12 Colby TV et al suggested that a TBLC sample with a diameter greater of 5 mm could be considered sufficient for pathologic analysis.13 In this case report the four samples retrieved had the largest diameter variable from around 1,2 cm to 5 mm and the total area was almost 200 mm2, allowing recognition of centrilobular areas and peripheral structures of the secondary pulmonary lobule.
In conclusion this case report suggests that TBLC might be considered as an another diagnostic step in those patients with increased pulmonary artery pressure and a significant low DLCO, and that, when a significant area of lung parenchyma is sampled, it might be a determinant in the diagnosis of ultra-rare lung disorders such as PCH/VOD.14