Paracoccidioidomycosis is a systemic mycosis which is endemic in rural areas of Latin America, an important European source of immigrants and a growing European touristic destination as well, with most cases occurring in Brazil, Argentina, Venezuela and Colombia. The authors report a case of a 43-year-old man who previously worked in Venezuela and is living in Portugal for 8 years, presenting with a single cutaneous lesion. Despite the absence of valuable respiratory complaints, severe lung damage was found with high-resolution computed tomography (HRCT). Biopsy of the cutaneous lesion and mycologic sputum examination were performed revealing Paracoccidioides brasiliensis infection.
A Paracoccidioidomicose é uma micose sistémica endémica nas áreas rurais da América Latina, uma fonte importante de imigrantes e destino de emigração e turismo europeu, a maioria dos casos ocorrendo no Brasil, Argentina, Venezuela e Colômbia. Os autores descrevem o caso clínico de um paciente com 43 anos, anteriormente emigrado na Venezuela e residente em Portugal há 8 anos, que se apresenta com lesão cutânea isolada. Embora sem queixas relevantes do foro respiratório, apresentava extensas lesões do parênquima pulmonar caracterizadas por tomografia computorizada de alta resolução (TCAR). Foi realizada biópsia da lesão cutânea e exame micológico da expetoração que revelaram infeção por Paracoccidioides brasiliensis (PB).
Paracoccidioidomycosis (PCM) is the most common endemic systemic mycosis in Latin America. It occurs in about 10% of the population in the subtropical regions of Brazil,1 and mostly affects farm workers; the highest incidence happens between the ages of 25 and 60.2 The causing agent, Paracoccidioides brasiliensis (PB), is a dimorphic fungus that can remain viable in the host for long periods, maintaining the potential for disease reactivation for several years after the initial infection. The portal of entry is the respiratory tract, the lung being the organ which is most frequently affected.1 Chronic infection with severe lung damage and progression to end-stage fibrosis occurs even where there are no obvious symptoms. Spread from a primary pulmonary lesion may affect other organs, not only the skin and mucous membranes most often, but also the adrenal glands, kidneys, gastrointestinal tract, liver, spleen and central nervous system.3,4 The most common sequelae include fibrosis with respiratory failure, cor pulmonale and also Addison's disease and intestinal malabsorption.2 The authors present a case of PCM with pulmonary and cutaneous involvement.
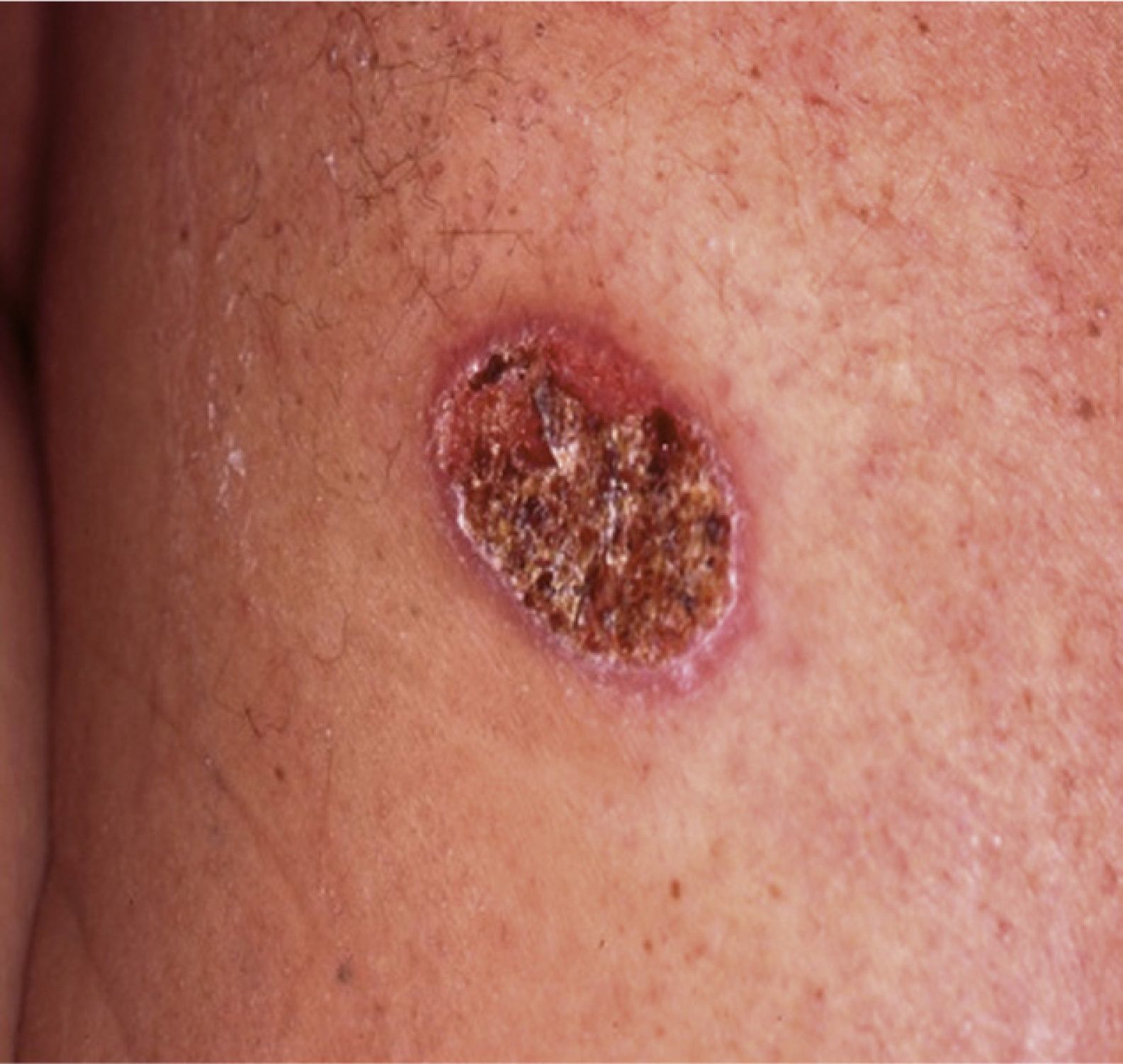
Case reportA 43-year-old Portuguese male, former farm worker in Venezuela until 2001, presented with a single back ulcerated lesion, with a 2-month evolution (Fig. 1). He had no other complaints and his clinical background was unremarkable, except for a previous ulcerated lesion of the nasal mucosa in 1996, for which he had had an unspecified 6-month treatment. His general condition was normal, without fever, palpable lymphadenopathy or hepatosplenomegaly. There were no laboratory changes, results for HIV 1 and 2 were negative. Bilateral diffuse changes in pulmonary auscultation were noticed and a chest radiograph was performed, showing a bilateral reticulo-nodular pattern predominantly in the middle and upper lung zones and a segmental consolidation in the right lung base.
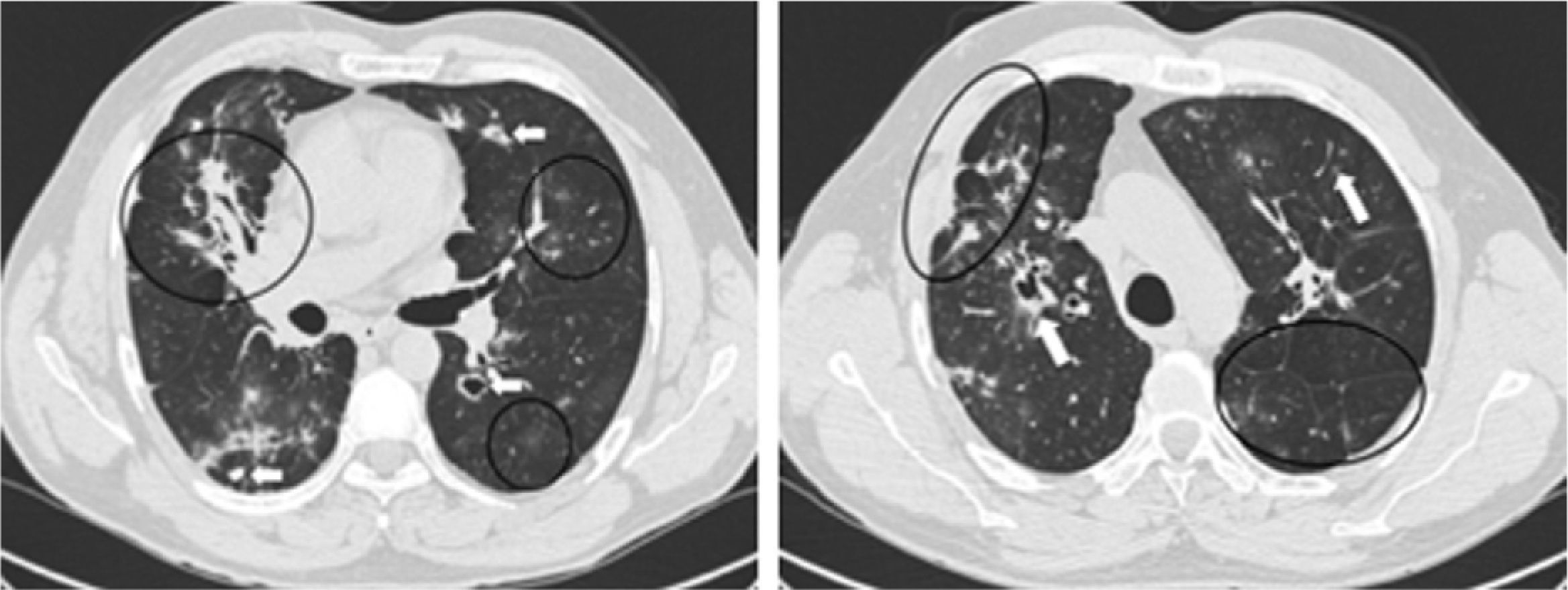
Pulmonary HRCT revealed exuberant bilateral parenchymal lesions with fibrotic changes and areas of active inflammatory disease mostly affecting the middle and upper lobes, despite the absence of respiratory symptoms. Multiple irregular spiculated nodular lesions ranging from 8 to 25mm in size were identified, some with central cavitation and confluence; these were most conspicuous in the upper lobes. Other findings included a central area of consolidation with air bronchogram in the middle lobe, architectural distortion, scattered foci of ‘ground glass’ attenuation, septal thickening, parenchymal bands, diffuse pleural speculated thickening, apical emphysematous bubbles and traction bronchiectasis. Hilar adenopathy and parietal irregularity of a dilated trachea were also present (Fig. 2).
Pulmonary HRCT findings. Left: Middle lobe consolidation with air broncogram and architectural distortion. Scattered ‘ground glass’ pattern through the left lung. Bilateral granulomas, some with cavitation (arrows). Mediastinal pleura spiculated thickening. Right: Right upper lobe paracicatricial enphysema and cavitation. Left lung parenchymal band (arrow) and interlobular septal thickening.
Biopsy of skin lesions and mycological examination of sputum revealed infection by Paracoccidioides brasiliensis (PB).
Therapy with itraconazole (200mg/day for 2 months, followed by 100mg/day for 8 months) was performed, with favorable clinical course.
DiscussionPCM was initially described by a Brazilian doctor, Lutz, in 1908.2 It is the most common endemic mycosis in Latin America. Brazil has the highest number of cases; it is most prevalent in subtropical regions where it affects about 10% of the population.1,2
It is a systemic granulomatous disease, predominantly pulmonary and mucocutaneous. The lungs are involved in 50–100% of cases.1 The main route of infection is by inhalation of PB spores which are present in the soil.1,2,4,7,8 The host's initial contact with the fungus usually progresses to a subclinical infection, with formation of a pulmonary granuloma, which may resemble the primary complex of tuberculosis.1,2
In most individuals the natural defense mechanisms provide a balance between the host and the agent, with the fungus remaining viable in a latent form.1,3,4,6 Spread to other organs and tissues may occur, most often to the skin and the mucosa of the airway and the oral cavity, with formation of granulomatous hemorrhagic ulcerated lesions.5
Two clinical forms have been described:
- -
an acute/subacute or juvenile type, accounting for less than 10% of cases, affecting both sexes under the age of 25 years, with fever, weight loss and malaise. There is rapid and progressive organ involvement with diffuse superficial and deep lymphadenopathy and hepatosplenomegaly. In a small number of cases cutaneous and osteolytic lesions coexist. The small intestine is involved in about 50% of cases. There is rarely involvement of the lung and bone marrow. The most common complications are lymphatic obstruction, intestinal malabsorption or protein-losing enteropathy.2,4
- -
a chronic form that arises primarily in adult males (male / female ratio of 10:1–25:1), 25–60 years old, most often involving the lung, followed by skin and mucous membranes. Patients may be asymptomatic or present with dyspnea, cough and occasionally hemoptysis. Fever is rare and the physical examination is often normal. This lack of respiratory symptoms may be paradoxical compared to the exuberance of pulmonary lesions. Morbidity results especially from the development of respiratory failure and cor pulmonale.1,2,4,6 Spontaneous pneumothorax is rarely the first clinical manifestation.6
The chest radiograph in PB infection may reveal linear and reticular opacities, differently-sized nodules, poorly defined infiltrates, areas of airspace consolidation and cavitation.6 This examination, however, has limited capacity for evaluating diffuse lung disease so HRCT has become the preferred method for evaluating patients with clinical suspicion and / or laboratory evidence of pulmonary PCM.
The changes in the lung parenchyma usually display a bilateral symmetric distribution, affecting the whole lung but predominantly the middle and upper lobes.7–10 Both active phase injuries and chronic fibrotic changes may be found simultaneously.
Lung disease is manifested on HRCT by consolidation, ‘ground glass’ opacities, scattered parenchymal nodules (granulomas or paracoccidiomas), septal thickening, spiculated pleural thickening, bronchial wall thickening, traction bronchiectasis, tracheal dilation, architectural distortion, parenchymal bands, and paracicatricial emphysema.7–10 Hilar and mediastinal lymphadenopathy are often present.9,10
Parenchymal consolidation represents a focal pneumonia, which usually starts with a desquamative alveolitis (associated with ‘ground glass’ opacities) and consists of an inflammatory infiltrate rich in fungi.9 The ‘reversed halo’ sign (central ‘ground glass’ pattern surrounded by crescent or ring-shaped peripheral consolidation), appears in about 10% of patients with active infection.11,12
Granulomas are characterized by irregular, spiculated margins and variable dimensions, assuming bizarre forms and a tendency to confluence. Cavitation is common, reflecting exudative inflammation.9,10
Pulmonary fibrosis is a common finding reflected by thickening of inter and intralobular septa, thickening of bronchial walls, architectural distortion, parenchymal bands and irregular thickening of the perihilar axial interstitium, involving hilar lymph nodes and main bronchi and pulmonary artery branches. This may be responsible for the development of cor pulmonale.1,7–10
The definitive diagnosis of this mycosis is obtained where PB is observed in biological fluids or tissues, mainly by direct mycological and/or histological examination or by serological techniques (antibody titers) that are also useful in assessing response to treatment and detection of recurrence.2,4,5
Active clinical disease can be treated with trimethoprim-sulfometoxazol or itraconazole. The most severe cases of chronic or acute illness can be treated with amphotericin B.2,12,13
ConclusionsAlthough uncommon in everyday clinical practice, PCM should be considered as a diagnostic hypothesis when there has been potential exposure, even if not recent, in endemic areas. High Resolution CT is essential for diagnosing these patients, as it accurately assesses the degree of lung parenchymal involvement.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Armas, M; Paracoccidioidomicose pulmonar: relato de caso clínico com aspectos em tomografia computorizada de alta resolução. Rev Port Pneumol 2012. doi:10.1016/j.rppneu.2012.02.001.