Determining the risk of recurrence of primary spontaneous pneumothorax is challenging. The objective of this study was to develop a risk assessment model to predict the probability of recurrence in patients with spontaneous pneumothorax.
MethodsA retrospective study was performed of all episodes of pneumothorax diagnosed in the last 12 years in a hospital, in patients not initially submitted to surgery. Logistic regression was used to estimate the probability of recurrence. Based on a set of variables, a predictive model was built with its corresponding ROC curve to determine its discrimination power and diagnostic precision.
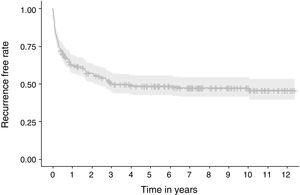
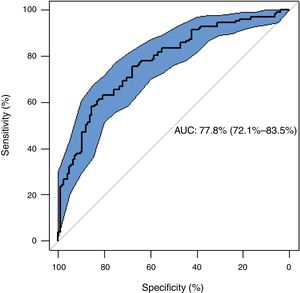
ResultsOf the 253 patients included, 128 (50.6%) experienced recurrence (37% within the first year). Recurrence was detected within 110 days in 25% of patients. The median of time to recurrence for the whole population was 1120 days. The presence of blebs/bullae was found to be a risk factor of recurrence (OR: 5.34; 95% CI: 2.81–10.23; p=0.000), whereas chest drainage exerted protective effect (OR: 0.19; 95% CI: 0.08–0.40; p=0.000). The variables included in the regression model constructed were hemoglobin and leukocyte count in blood, treatment received, and presence of blebs/bullae, with a fair discriminative power to predict recurrence [AUC=0.778 (95% CI: 0.721–0.835)].
ConclusionThe overall recurrence rate was high and was associated with the presence of blebs/bullae, failure to perform an active intervention (chest drainage) and low levels of hemoglobin and leukocytes in blood. Recurrence rarely occurs later than three years after the first episode. Once validated, this precision model could be useful to guide therapeutic decisions.
Spontaneous pneumothorax is one of the most common pleural disorders, and around 86% correspond to idiopathic pneumothorax.1 The annual incidence of primary spontaneous pneumothorax (PSP) is 18–28 cases/100,000 in men, and 1.2–6/100,000 in women2 and this disease generally affects young patients. Recommended treatment for large PSP is active intervention (needle aspiration or chest drainage with small drain tubes). Surgery is generally reserved for episodes of pneumothorax that do not resolve, recurrences, pneumothorax associated with hemothorax, bilateral pneumothorax or risk occupations.2–4
There is considerable variation in the rates of recurrence of PSP reported in the literature. In a systematic review of 29 articles (4 randomized clinical trials and 25 observational studies) involving more than 13,500 patients, the overall rate of recurrence was 32%,5 ranging between 8%6,7 and 74%.8 This wide range makes it difficult for physicians to determine the actual risk of recurrence and select the most effective therapy. Assuming a rate of recurrence close to the lowest estimates, it would seem reasonable to wait for a second episode prior to considering surgery. In contrast, if the highest estimates are accepted, surgery should be considered after a first episode of pneumothorax.
No factors have been identified to date as predictors of recurrence in PSP. As a result, the risk of recurrence cannot be estimated for a particular patient. Female gender, low body weight, smoking, and height in men have been postulated as recurrence risk factors.9–12 A set of radiological findings such as bullae/blebs on CT and pleural thickening on chest X-ray have also been associated with a higher risk of recurrence.8,13,14 To date, no treatment has been associated with a lower risk of recurrence.15–18
The objectives of this study were to estimate the rate of recurrence of PSP diagnosed in our hospital in the last 12 years and managed with a medical treatment (both, during the first year and by the end of the inclusion period). Another objective was to identify factors associated with recurrence, develop an individual-risk assessment model, and determine whether a specific approach is associated with a lower rate of recurrence.
Material and methodsDesignA retrospective study was conducted in patients diagnosed with PSP managed with medical treatment between January 2007 and December 2018 in a tertiary 1000-bed hospital serving a population of 450,000.
Data collectionDischarge reports were searched for the International Classification of Disease, 9th and 10th revision codes consistent with PSP (ICD-9-CM and ICD-10-CM).
DefinitionsPSP: first episode of the presence of air in the pleural space not associated with a known pulmonary disease, previous trauma or medical treatments. Recurrence of PSP: second ipsilateral episode or first contralateral episode of PSP. Medical treatment: a therapeutic approach based on observation, aspiration or chest drainage. Blebs and bullae: the presence of subpleural air spaces with thin walls measuring <1 and ≥1cm, respectively. Non-smoker: subjects who have smoked less than 100 cigarettes in their lifetime or have never smoked. Ex-smoker: subjects who quitted smoking at least 6 months earlier.
Selection criteriaEligible cases were adults (≥16 years) admitted for a first episode of PSP between 2007 and 2018, inclusive. Patients were excluded if they had secondary spontaneous pneumothorax (infections, neoplasms, chronic obstructive pulmonary disease, diffuse interstitial pulmonary disease like hystiocitosis X or lymphangioleiomyomatosis, among other); traumatic; iatrogenic pneumothorax; if pneumothorax had been initially treated surgically (thoracoscopy or pleurodesis); if they were <16 years, and if all required data were not available.
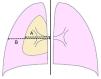
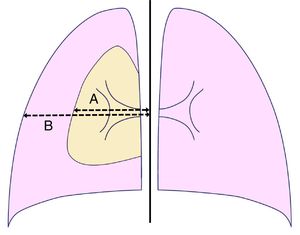
DatabaseA database was built including the following factors: demographic [age, gender, weight, height, body mass index (BMI) (weight (in kg)/height2 (in meters)]; clinical [smoking habit (pack-year index) and approach adopted [observation, Heimlich valve, aspiration, chest drainage (tube gauge, drain duration), etc.]; analytical (complete hemogram, coagulation tests, liver and renal function tests); and radiological data [presence of bullae/blebs, laterality, lung height (at maximum inspiration), size of the pneumothorax according to Light's index [size of the pneumothorax (in %)=[(1−(L3/HT3)]×100, where L and HT are the diameters of the lung and the hemithorax, respectively, both measured at the level of the pulmonary hilum] (Fig. 1),19 and the relationship between the maximum transverse and anteroposterior internal sizes of the thorax on chest X-ray (distance between the internal surface of the ribs in the two sides and between the internal surface of the sternum and the anterior face of the vertebral body, respectively), measured at the level of the xiphoid apophysis]. Images were obtained using the digital AXIUM ARISTOS TX (Siemens) system and processed using the IDS7 work station (Sectra). Radiological measurements were performed separately by two experienced operators blind to other data. Discrepancies (presence or not of bullae/blebs and distance differences >5%) were discussed and solved by consensus.
The study was approved by the Ethics Committee of the hospital (CEIC 2019/008).
Statistical analysisLogistic regression and survival models (time to recurrence) were built to estimate the probability of recurrence. The variables listed above found to be associated with recurrent pneumothorax were included in the models. All variables were included in the initial model. Then, the variables with the lowest weight according to the likelihood ratio were removed. Only significant variables were included in the final model (p<0.05). Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated from logistic regression coefficients. A classification rule was built to estimate the probability of recurrence. ROC curves were constructed to evaluate the discrimination power of the model. Areas under the curve (AUC) were calculated with their 95% CI. Based on the predicted probabilities obtained from the models, the optimal cut-off for recurrent pneumothorax was calculated using Youden's index.20 Also, to calculate the optimal cut-off, sensitivity, specificity, predictive values and likelihood ratio were calculated with their 95% CIs. All statistical analyses were carried out in R using “survival”, “rms”, “pROC” and “OptimalCutpoints” packages. These packages are freely available at cran.r-project.org.
ResultsA total of 1622 patients were diagnosed with pneumothorax in our hospital during the study period. Fig. 2 shows the patient flow chart. The final number of patients with PSP included in the study was 253, of whom 128 (50.6%) experienced relapse during the follow-up period (overall recurrence rate). The recurrence rate during the first follow-up year was 37%. Recurrence occurred in the ipsilateral side in 98 patients (76.6%) and in the contralateral side in 30 (23.4%). The duration of follow-up ranged from 7 days (shortest time to recurrence) to 12 years (median, 26 months).
Table 1 displays the clinical characteristics of the totality of patients and study groups and provides data on the occurrence of recurrence. Raw OR are also shown. Significant differences were observed in age, smoking habits, presence or blebs/bullae, Light's index, transversal and anteroposterior chest size, hemoglobin, hematocrit and leukocyte count in blood, treatment received (observation vs chest drainage) and drainage duration. Factors found to have protective effects against recurrence included age, history of smoking habits, pack-year index, Light's index, chest drainage, transverse and anteroposterior size of the thorax; and hemoglobin, hematocrit and leukocyte count in blood. The protective value of Light's index disappeared with adjustment for treatment, as a high proportion of pneumothorax with a low Light's index were managed only with observation, whereas all pneumothorax with a high Light's index were drained. In contrast, the presence of blebs/bullae and a long chest drainage duration were identified as risk factors of recurrence.
Clinical and demographic characteristics of the patients included in the study and odds ratio of the factors that influence the recurrence of primary spontaneous pneumothorax.
| Variable | Total | No recurrence | Recurrence | OR (95% CI) | p |
|---|---|---|---|---|---|
| N | 253 | 125 | 128 (50.6) | ||
| Men (%) | 199 (79) | 97 (48.7) | 102 (51.3) | 0.71 (0.38, 1.29) | 0.259 |
| Age (mean) | 25.9±8.5 | 27.3±8.1 | 24.6±8.7 | 0.96 (0.93, 0.99) | 0.011 |
| Body mass index [weight (kg)/height2(in meters)] | 21.3±2.9 | 21.6±2.8 | 20.9±3 | 0.92 (0.83, 1.01) | 0.079 |
| Smokers | |||||
| Never-smokers (%) (Ref) | 102 (43.8) | 39 (38.2) | 63 (61.8) | ||
| Smokers (%) | 120 (51.5) | 66 (55) | 54 (45) | 0.51 (0.30, 0.87) | 0.012 |
| Ex-smokers (%) | 11 (4.7) | 8 (72.7) | 3 (27.3) | 0.23 (0.06, 0.93) | 0.039 |
| Packs-year | 9.4±6.9 | 9.8±6.6 | 9±7.2 | 0.95 (0.91, 0.99) | 0.566 |
| Right-side pneumothorax | 139 (54.9) | 73 (52.5) | 66 (47.5) | 1.32 (0.80, 2.17) | 0.274 |
| Blebs/bullae | 186 (73.5) | 73 (39.2) | 113 (60.8) | 5.34 (2.81, 10.23) | 0.000 |
| Light's index | 44.1±27.6 | 48±26 | 40±28 | 0.99 (0.98, 0.99) | 0.004 |
| Transverse size of the thorax (cm) | 29±2.5 | 29.3±2.4 | 28.6±2.6 | 0.90 (0.81, 0.99) | 0.042 |
| Antero-posterior size of the thorax (cm) | 12.5±2 | 12.9±2 | 12.1±1.9 | 0.82 (0.72, 0.93) | 0.002 |
| Transverse/anteroposterior size coefficient | 2.4±0.3 | 2.3±0.3 | 2.4±0.4 | 2.23 (1.06, 4.67) | 0.032 |
| Height of the lung (cm) | 25.2±2.2 | 25.3±2.1 | 25.1±2.2 | 0.95 (0.85, 1.07) | 0.429 |
| Hemoglobin (g/dL) | 14.9±1.2 | 15.1±1.1 | 14.7±1.2 | 0.75 (0.60, 0.93) | 0.009 |
| Hematocrit (%) | 43.4±3.4 | 44±3.2 | 42.9±3.4 | 0.90 (0.84, 0.97) | 0.008 |
| Leukocytes (cells×103/μL) | 9.8±3.2 | 10.5±3.3 | 9.2±3 | 0.87 (0.81, 0.95) | 0.001 |
| Platelets (cells×103/μL) | 250.1±59 | 258.4±61.3 | 244±56.2 | 0.99 (0.99, 1.00) | 0.096 |
| Treatment | |||||
| Observation (%) (Ref) | 37 (14.6) | 7 (18.9) | 30 (81.1) | ||
| Thoracic drainage (%) | 216 (85.4) | 118 (54.6) | 98 (45.4) | 0.19 (0.08, 0.46) | 0.000 |
| Diameter of chest tube [<20F (%)] | 75 (36.2) | 37 (49.3) | 38 (50.7) | 0.72 (0.41, 1.27) | 0.252 |
| Drainage duration (days) | 3.1±2.1 | 2.7±1.8 | 3.5±2.4 | 1.20 (1.04, 1.37) | 0.008 |
F, French; OR, odds ratio; 95% CI, confidence intervals; Ref, reference category.
Data are expressed as means±standard deviation or in absolute frequencies (percentages).
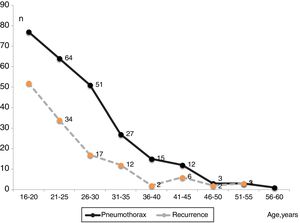
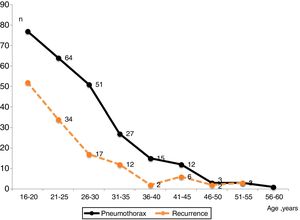
Fig. 3 shows the number of PSP cases and recurrences and the age at which they occurred. Fig. 4 displays the rate of patients who remained free of recurrence during follow-up (Kaplan–Meier curve). In total, recurrence was detected within 110 days in 25% of patients. The median of time to recurrence for the whole population was 1120 days (∼3 years). Thereafter, only 4.7% of patients experienced a relapse.
Table 2 shows the regression model constructed to predict the risk of recurrence of PSP. The variables included in the final model were hemoglobin and leukocyte count in blood, the approach (thoracic drainage or not) and presence of blebs/bullae on chest X-ray. The model demonstrated a good discriminative power for the prediction of recurrence [AUC=0.778 (95% CI=0.721, 0.835)] (Fig. 5).
Logistic regression model for the prediction of relapse after a primary spontaneous pneumothorax.
| Coefficients | SE | OR (95% CI) | p | |
|---|---|---|---|---|
| Intercept | 6.6147 | 2.0120 | ||
| Hemoglobin | −0.3511 | 0.1296 | 0.70 (0.55, 0.91) | 0.007 |
| Leukocytes (*1000) | −0.1414 | 0.0482 | 0.87 (0.79, 0.95) | 0.003 |
| Treatment | −1.5756 | 0.4809 | 0.21 (0.08, 0.53) | 0.001 |
| Blebs/bullae | 1.8639 | 0.3639 | 6.45 (3.16, 13.16) | <0.001 |
OR, odds ratio; 95% CI, confidence intervals.
A calculator of the probability of recurrence of PSP, and the formula used to estimate it, is available in the supplementary documentation. This tool may help clinicians use the predictive model.
Table 3 summarizes the diagnostic performance of the logistic regression model after the optimal cut-off was calculated using Youden's index (60% probability). The model has a sensitivity of 58%, a specificity of 86%, and a rate of correct classification of 71%. Table 4 shows sensitivity and specificity with different cut-off points of the predicted probability of PSP recurrence.
Classification table obtained using a logistic regression model for the prediction of relapse after pneumothorax.
| Test: | Recurrence | Total | |
|---|---|---|---|
| Yes | No | ||
| Positive | 96 | 43 | 252 |
| Negative | 31 | 82 | |
| Sensitivity (95% CI) (%) | 58 (49, 67) | ||
| Specificity (95% CI) (%) | 86 (78, 91) | ||
| Positive predictive value (95% CI) (%) | 80 (71, 86) | ||
| Negative predictive value (95% CI) (%) | 67 (58, 80) | ||
| Positive likelihood ratio (95% CI) | 4.04 (2.57, 6.36) | ||
| Negative likelihood ratio (95% CI) | 0.49 (0.39, 0.61) | ||
| Rate of correct classification (95% CI) (%) | 71 (65, 76) | ||
Optimal cut-off (Youden's index), 60%.
95% CI, confidence intervals.
Sensitivity and specificity at different cut-off point to predict relapse after a primary spontaneous pneumothorax with the model built.
| Cut-off | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| 10 | 99 (96, 100) | 6 (0, 11) |
| 20 | 94 (89, 98) | 29 (21, 38) |
| 30 | 92 (86, 96) | 39 (31, 48) |
| 40 | 86 (79, 91) | 46 (37,55) |
| 50 | 76 (67, 83) | 66 (57, 74) |
| 60 | 57 (48, 66) | 86 (78, 91) |
| 70 | 35 (27, 44) | 94 (88, 97) |
| 80 | 24 (17, 33) | 98 (94, 100) |
| 90 | 7 (3, 13) | 99 (96, 100) |
95% CI, confidence intervals.
The results obtained indicate a high rate of PSP recurrence (50.6%) and demonstrate that this prediction model has a fair discriminative power to predict recurrence based on the AUC obtained. Also, our results confirm that the presence of blebs/bullae on chest X-ray and the lack of an active intervention (chest drainage) in a first episode of pneumothorax increase the risk of recurrence, which rarely occurs later than three years after the first episode.
The overall rate of recurrence (50.6%) is consistent with that reported by other authors.9–11,13 To the best of our knowledge, this is the first study to propose a model to predict PSP recurrence, although some factors had already been associated with a higher risk of recurrence. This is of relevance, as the ability to determine the risk of recurrence of a PSP may determine the therapeutic approach to be adopted.
The rate of recurrence of PSP has been reported to be higher in women,5,9,11 while in our study no gender-based differences were observed. The reason may be that we exclude catamenial pneumothorax or underlying gender-related diseases such as lymphangioleiomyomatosis by not considering them PSP. Although age is not considered a risk factor for recurrence,9,10 in our study, recurrence was more frequent in younger patients (p=0.011). There is no consensus on the relationship between BMI and recurrence,9 yet some studies have found some evidence.10,11 Our results show that patients who relapsed had a lower BMI, although differences were not significant (p=0.079).
PSP destroys the lung parenchyma, and the increase in low attenuation areas in PSP patients is related to smoking.21 This suggests that patients with pneumothorax are more predisposed to the deleterious effects of tobacco. Nevertheless, there is limited evidence on the relationship between smoking and PSP recurrence.22 Some studies have revealed a higher tendency to relapse among non-smokers.9–11,13 In our study, recurrence was significantly higher among non-smokers (61.8%), as compared to smokers (45%) (p=0.012). Thus, the risk of recurrence in smokers was half that of non-smokers (OR 0.51; 95% CI: 0.30–0.87). This inconsistency of results may be due to the detrimental effect of smoking being obscured by the high baseline rates of cigarette smoking in the included studies and the heterogeneous classifications used to define smoking status.5
The probability of recurrence of PSP was five-fold higher when blebs/bullae were observed on X-ray after a first episode of pneumothorax (OR 5.34; 95% CI: 2.81–10.23; p=0.000). This association has been consistently reported in the literature, regardless of the imaging technique used (X-ray,13 or CT as in our study14). Although CT has higher sensitivity to detect pneumothorax, in most cases it is not required for a diagnosis to be established. Remarkably, routine CT use is not recommended in clinical guidelines2–4 to avoid the exposure of young patients to radiation.23 An initial CT is not performed in routine clinical practice in our hospital either. The presence of blebs/bullae is known to be very frequent in PSP, although some authors have not found any evidence of their association with a higher risk of recurrence.17,24
The morphology of the thorax has also been linked to a higher probability of PSP. Park et al. documented that the chest of patients with PSP is flatter in the anteroposterior view, narrower in the lateral view, and higher in the craniocaudal view. This indicates that the morphology of the chest is associated with the development of pneumothorax.25 Nevertheless, no studies have been published to establish a relationship between chest morphology and pneumothorax recurrence. In our study, recurrence was less frequent in patients with a larger size of the lung in the transverse and anteroposterior view (OR 0.90; 95% CI: 0.81–0.99; p=0.042 and OR 0.82; 95% CI: 0.72–0.93; p=0.002, respectively), which suggests that chest morphology may influence the recurrence of pneumothorax.
This study also reveals that lower levels of hemoglobin, hematocrit and leukocytes in blood and a low Light's index are risk factors for recurrent pneumothorax (Table 1). Nevertheless, the protective value of Light's index disappears with adjustment for treatment, as a high proportion of pneumothoraces with a low Light's index were only treated with observation, whereas all patients with a high Light's index underwent drainage. Thus, patients who underwent drainage were five-fold more likely to experience relapse that those whose pneumothorax was managed by observation (0.08–0.46; p=0.000). All in all, a long duration of chest drainage is associated with a higher risk of recurrence of pneumothorax (OR 1.20; 95% CI: 1.04–1.37; p=0.008). Although a recent study provides modest evidence that conservative management of PSP was noninferior to interventional management at 8 weeks follow-up, it does not address how many patients subsequently recurred in each group.26
Our predictive model is based on hemoglobin and leukocyte count in blood, the treatment administered (observation or thoracic drainage), and the presence of blebs/bullae on chest X-ray. The predictive power of the model is fair (AUC 0.778; 95% CI: 0.721–0.835), and its performance is optimized with a cut-off for the probability of recurrence of 60% (Table 4). We do not know any plausible biological hypothesis to justify the effect of the levels of these parameters on the recurrence of pneumothorax.
Given the overall rate of recurrence observed in our area (50.6%), it is recommended that patients are informed that 1 out of 2 PSP will relapse within a year (37%). This information, together with the probability of recurrence obtained using our predictive model for a particular patient can guide the therapeutic decision. Thus, a young patient with a long life expectancy and a high probability of recurrence will prefer to undergo an initial, more-aggressive treatment that spares them from the uncertainty of a potential relapse.27 A multicentric, one-year follow-up study assessing the clinical benefits of surgery in a first episode of PSP revealed that the rate of recurrence was significantly higher in patients treated with chest drainage as compared to those who underwent VATS (34% vs 13%, respectively). The study showed that five patients would have to undergo surgery to prevent a relapse.28 Therefore, treatments will be individualized in the future according to the clinical context.
This study has some limitations. First, it is a retrospective study, and the results obtained should be confirmed with new prospective studies. Secondly, the number of PSP was relatively low (253). Third, patients were recruited in a single center, and external validation with patients from other hospitals is required. Fourth, these results should not be applied to patients initially treated surgically (thoracoscopy or pleurodesis). Finally, estimating the probability of developing PSP requires complex calculations. However, the Excel calculator available in the supplementary material will make calculations easier for clinicians.
In summary, the overall rate of recurrence of PSP in our area is high and is associated with the presence of blebs/bullae on chest X-ray, the absence of an active intervention, and a low hemoglobin and leukocyte count in blood. Recurrence rarely occurs later than three years after the first episode. A predictive model of recurrence may help clinicians choose the most appropriate treatment. Larger, prospective, randomized, controlled trials and
cost-effectiveness studies are required to determine the most appropriate management approach in each case of PSP.
Ethical responsibilitiesNone declared.
FundingThis study was performed without funding.
Conflicts of interestThe authors have no conflicts of interest to declare.