Mycophenolate mofetil (MMF) is a cell cycle inhibitor widely used in patients with solid organ transplants1, in the treatment of many autoimmune diseases2,3 and, even, in hypersensitivity pneumonitis4. Its most frequent side effects are myelosuppression, gastrointestinal disorders and an increased susceptibility to infections, including pneumonia3,4. The development of interstitial lung disease after taking this drug is a rare adverse effect of which only isolated cases have been published5–7. We present a patient with pulmonary toxicity due to MMF.
A 72-year-old woman came to the Emergency Department after seven days of fever, asthenia and dyspnoea. In 2008, she had received a kidney transplant due to chronic renal failure secondary to Polycystic kidney disease and had begun treatment with corticosteroids, sirolimus and MMF (640mg/12hours). In 2014, a chest computed tomography (CT) scan revealed an extended micronodular interstitial lung disease that disappeared after withdrawal of sirolimus. It was interpreted as a pulmonary toxicity and was replaced by tacrolimus.
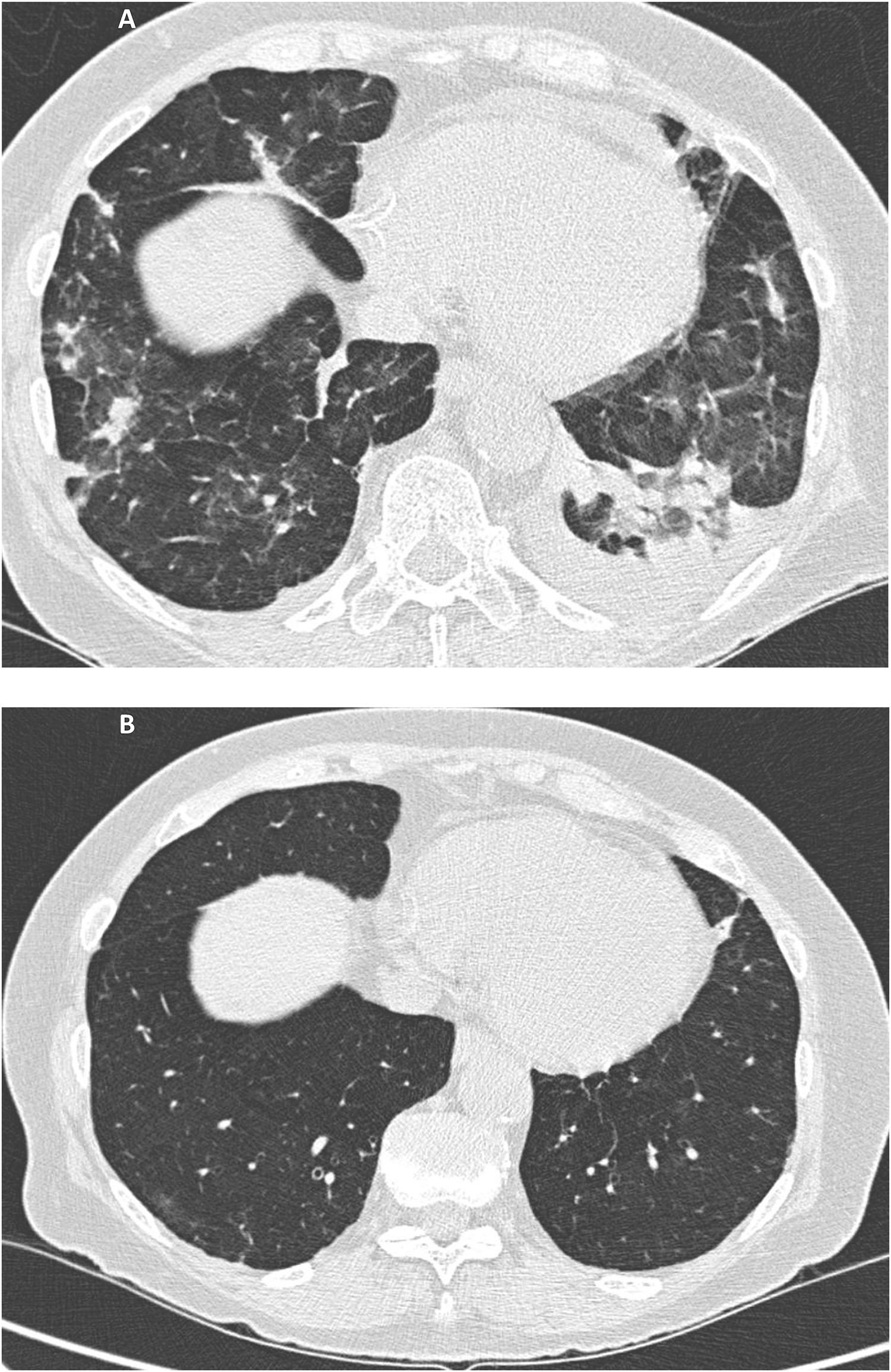
Upon arrival to the Emergency Room she had a fever (37.5°C) with diminished respiratory sounds and crackles in the middle of the left hemithorax. The patient had never smoked, had no close contact with animals and had not engaged in any potentially harmful professional, recreational or domestic activities. Blood test results showed hemoglobin 11.7g/dL, leukocytes 5,000/mm3 with lymphopenia (650/mm3; 13%), urea 70mg/dL, creatinine 1.57mg/dL and C-reactive protein 1.9mg/dL. Arterial blood gases evidenced hypoxemia (pO2 49.7mmHg) and chest CT demonstrated multifocal pulmonary lesions with areas of ground-glass and small bilateral pleural effusion of left predominance (Fig. 1A). The cultures in different biological samples (blood, sputum, bronchoalveolar lavage and pleural fluid) for bacteria, tuberculosis, virus (cytomegalovirus, influenza, parainfluenza, herpes, chickenpox, syncytial, HIV, etc.), aspergillus-galactomannan and pneumocystis jirovecii were all negatives. Serology against Coxiella, Legionella, Chlamydia, Toxoplasma and Mycoplasma pneumoniae was negative. Macroscopically, bronchofibroscopy was normal. Bronchoalveolar lavage showed a lymphocyte predominance (50%) with a CD4/CD8 T-lymphocyte ratio of 4.35. There were relevant alterations in the smear findings. The pleural fluid had biochemical exudate characteristics with 100% mononuclear cells and an adenosine deaminase of 14 IU/L. Echocardiography ruled out endocarditis. Neither pulmonary function tests nor any type of lung biopsy were performed due to the patient's clinical situation and the presumably limited collaboration. During the first days of admission, treatment with MMF and tacrolimus were maintained, and different antibiotic cycles were performed (levofloxacin, ceftriaxone, piperacillin/tazobactam, voriconazole, amikacin and linezolid), without improvement. The fact that all cultures were negative and the lack of response obtained led to rejection of the hypothesis of an infectious cause in an immunocompromised patient and to considering the possibility of pulmonary toxicity. Antibiotics and MMF were discontinued and tacrolimus was maintained. Since then, the clinical and radiological improvement was progressive, with resolution of fever, respiratory failure and radiological alterations, including pleural effusion (Fig. 1B). Once clinical improvement was observed, the oral corticosteroid dose of methylprednisolone was increased to 80mg/day. After nine months of follow-up, the patient is asymptomatic.
A Multiple small consolidations in both lungs with areas of ground glass opacification and small bilateral pleural effusion of left predominance. Fig. 1B. Complete resolution of the extensive pulmonary disease with consolidative component and ground glass, as well as the small bilateral pleural effusion.
MMF is used as a first or second immunosuppressant line in solid organ transplant protocols since it inhibits the purine biosynthesis of T and B lymphocytes through the inosine monophosphate dehydrogenase enzyme, limiting the proliferation of lymphocytes. In this way, there is a reduction in the generation of effector lymphocytes for T cell-mediated immunity and the production of antibodies. Its profile of adverse effects is acceptable. In all cases published to date, the appearance of interstitial lung disease induced by MMF manifested in the short-medium term (maximum 18 months) after the introduction of the drug7. However, this is the first case described in which lung disease appears after the use of MMF in the long term (9 years).
The development of interstitial lung disease with MMF is an unusual side effect of immunosuppression. The main difficulty in establishing a link between any immunosuppressive agent and a lung disease is that it is a diagnosis of exclusion. In this case, the temporal relationship, the clinical and CT characteristics of the thorax, the negative cultures and the symptomatic and radiological improvement after the removal of MMF suggest interstitial lung disease induced by MMF, although an atypical infection cannot be safely excluded. Cryptogenetic organizing pneumonia and pulmonary eosinophilia were ruled out when presenting clinical-radiological improvement with a minimum dose of corticosteroids and eosinophils were not demonstrated in bronchoalveolar lavage, respectively.
In summary, what can be learned from this case is that MMF is a drug that must be taken into account as a potential cause of interstitial lung disease. Although rare, this is a serious adverse effect. Therefore, in the presence of fever, dyspnoea and diffuse pulmonary involvement in chest CT, this possibility should be considered. An empirical test of MMF suspension and observation of symptomatic improvement could be enough to identify the drug as the probable cause of the disease.