Symptoms are the cornerstone for diagnosing acute exacerbations of chronic obstructive pulmonary disease (AECOPD), however little information is available on their variability during these events and on their relationships with objective clinical measures. This study explored changes in patients’ symptoms and their relationships with objective clinical measures during AECOPD.
MethodsA longitudinal observational study was conducted with thirty-six outpatients with AECOPD (24 males; 68.4±9.9 years; forced expiratory volume in one second (FEV1) 50.7±20.4%predicted) recruited from the urgent care of a Central hospital. Patients attended to 4 assessments: until 48hours of the urgent care visit (T1), 8 days (T2), 15 days (T3) and 45 days (T4) after the hospital visit. Patients’ prescriptions included only pharmacological treatment and consisted in antibiotics (n=16; 44.4%), beta-adrenergic agonists (n=2; 5.6%), cholinergic antagonists (n=3; 8.3%), associations of bronchodilators with cholinergic antagonists (n=7; 19.4%), anti-inflammatory drugs (n=1; 2.8%), xanthines (n=1; 2.8%) and expectorants (n=6; 16.7%).
Activities-related dyspnoea (modified British Medical Research Council questionnaire – mMRC), dyspnoea and fatigue at rest (modified Borg Scale – MBS), cough, sputum and wheezing symptoms (11-point numerical scale) were registered in each assessment. FEV1, using a portable spirometer, and quadriceps muscle strength (QMS), using a handheld dynamometer, were also collected.
The number of participants presenting symptoms, the severity of symptoms, FEV1 and QMS were compared among T1, T2, T3 and T4 using the Cochran or Friedman tests, respectively. Changes in symptoms were correlated with changes in FEV1 and QMS using the Spearman's correlation coefficient.
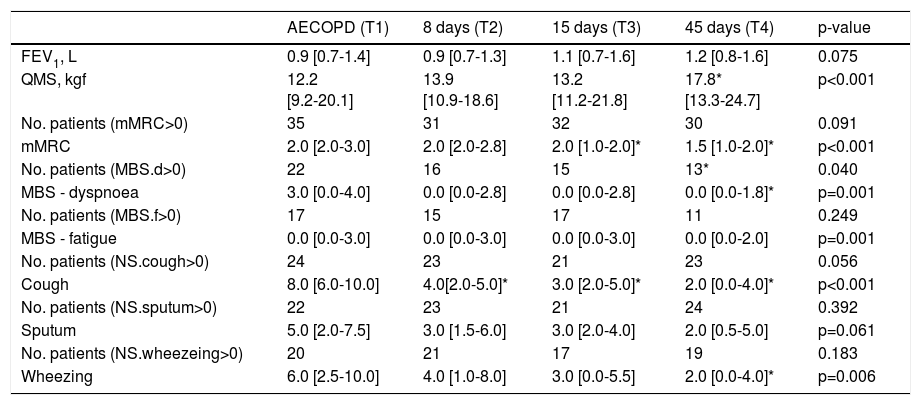
ResultsDyspnoea and cough were the most reported symptoms at the onset of AECOPD. The number of patients with dyspnoea at rest, assessed with the MBS (MBS>0), decreased significantly from T1 to T4 (22 vs. 16 vs. 15 vs. 13; p=0.040) (Table 1). No significant differences were observed in the number of patients presenting activities-related dyspnoea, fatigue at rest, cough, sputum and wheezing symptoms. During the time course of the AECOPD, participants presented significantly more i) activities-related dyspnoea in T1, than in T3 (p=0.001) and T4 (p=0.028); ii) dyspnoea at rest in T1 than in T4 (p=0.016); iii) cough in T1 than in T2 (p=0.001), T3 (p<0.001) and T4 (p<0.001) and iii) wheezing in T1 than in T4 (p=0.022) (Table 1).
Clinical variables and symptoms variability during the course of an AECOPD.
| AECOPD (T1) | 8 days (T2) | 15 days (T3) | 45 days (T4) | p-value | |
|---|---|---|---|---|---|
| FEV1, L | 0.9 [0.7-1.4] | 0.9 [0.7-1.3] | 1.1 [0.7-1.6] | 1.2 [0.8-1.6] | 0.075 |
| QMS, kgf | 12.2 [9.2-20.1] | 13.9 [10.9-18.6] | 13.2 [11.2-21.8] | 17.8* [13.3-24.7] | p<0.001 |
| No. patients (mMRC>0) | 35 | 31 | 32 | 30 | 0.091 |
| mMRC | 2.0 [2.0-3.0] | 2.0 [2.0-2.8] | 2.0 [1.0-2.0]* | 1.5 [1.0-2.0]* | p<0.001 |
| No. patients (MBS.d>0) | 22 | 16 | 15 | 13* | 0.040 |
| MBS - dyspnoea | 3.0 [0.0-4.0] | 0.0 [0.0-2.8] | 0.0 [0.0-2.8] | 0.0 [0.0-1.8]* | p=0.001 |
| No. patients (MBS.f>0) | 17 | 15 | 17 | 11 | 0.249 |
| MBS - fatigue | 0.0 [0.0-3.0] | 0.0 [0.0-3.0] | 0.0 [0.0-3.0] | 0.0 [0.0-2.0] | p=0.001 |
| No. patients (NS.cough>0) | 24 | 23 | 21 | 23 | 0.056 |
| Cough | 8.0 [6.0-10.0] | 4.0[2.0-5.0]* | 3.0 [2.0-5.0]* | 2.0 [0.0-4.0]* | p<0.001 |
| No. patients (NS.sputum>0) | 22 | 23 | 21 | 24 | 0.392 |
| Sputum | 5.0 [2.0-7.5] | 3.0 [1.5-6.0] | 3.0 [2.0-4.0] | 2.0 [0.5-5.0] | p=0.061 |
| No. patients (NS.wheezeing>0) | 20 | 21 | 17 | 19 | 0.183 |
| Wheezing | 6.0 [2.5-10.0] | 4.0 [1.0-8.0] | 3.0 [0.0-5.5] | 2.0 [0.0-4.0]* | p=0.006 |
Legend: Values are shown as number or median [interquartile range]; significant difference at p<0.05; * different from T1.
FEV1, forced expiratory volume in one second; mMRC, modified British Medical Research Council questionnaire; MBS.d, modified Borg scale – dyspnoea; MBS.f, modified Borg scale – fatigue; NS, numerical scale; QMS, quadriceps muscle strength.
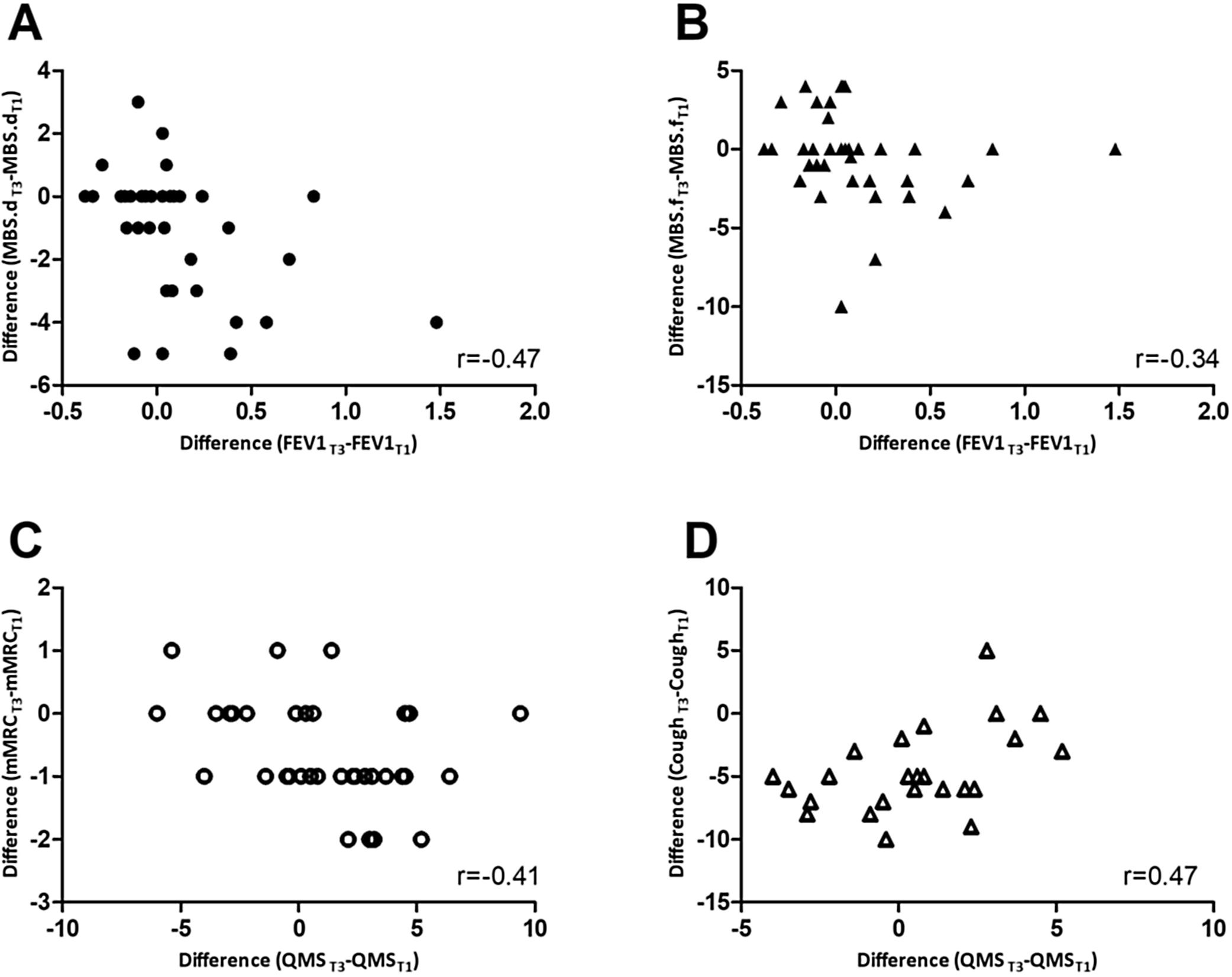
Changes occurring between T1 and T3 in mMRC correlated inversely with changes in QMS (rs=-0.41; p=0.013) whilst changes in cough (rs=0.47; p=0.021) correlated positively with QMS. Changes in MBS – dyspnoea (rs=-0.47; p=0.004) and fatigue (rs=-0.34; p=0.046) correlated inversely with changes in FEV1 (Fig. 1). No further correlations were found.
Correlations between changes from T1 to T3 in A) modified Borg scale – dyspnoea (MBS.d) and forced expiratory volume in 1 second (FEV1); B) modified Borg scale – fatigue (MBS.f) and FEV1; C) modified British Medical Research Council questionnaire (mMRC) and quadriceps muscle strength (QMS); D) Cough, assessed with the numerical scale, and QMS.
Dyspnoea and cough were the most reported symptoms at the onset of AECOPD.1,2 Dyspnoea was the most prevalent symptom. Its time-recovery matched previous reports (i.e, 6 to 30 days).1,2 Cough was the symptom reported with the highest severity and the first to improve after treatment initiation. In COPD cough is the most common symptom for which individuals seek medical attention and is a cardinal symptom in upper tract infections,3 one of the most common causes of AECOPD. Our results support the need of increasing awareness about cough severity and behaviour. Recognising the cough pattern may aid to guide patients’ monitoring and interventions, reduce need for hospitalisation, recurrence of AECOPD and, consequently, costs and morbidity related with these events.
Differences in wheezing were only detected 45 days after the onset of the exacerbation, which differs from previous reports using computerised respiratory sound analysis (i.e., improvements 15 days after the AECOPD).4 Lack of agreement between subjective and objective measures have already been reported for other outcomes, such as cough,5 and highlights the need for incorporating both patient-reported and clinical outcome measures in the assessment of patients with AECOPD.
Similar to other studies, associations between improvements in dyspnoea and higher expiratory flow rates were found, possibly due to the inflammatory aetiology of the acute exacerbation itself (i.e., reduction in inflammation during recovery from the AECOPD may influence the reduction of dyspnoea and increase expiratory flow rates) and/or reductions in lung hyperinflation.2 Nevertheless, both inflammation and hyperinflation were not directly studied in the present research and thus interpretations should be made carefully. A relationship between dyspnoea and QMS was also found, as previously reported in stable patients with COPD, due to the “downward disease spiral” of increased dyspnoea, decreased physical activity and deconditioning of locomotor muscles.6 During AECOPD, this downward spiral may be even more prominent as patients severely decrease their activities.
The positive correlation found between changes in cough severity and QMS was unexpected. Whilst cough severity showed significant improvements at day 15 of the AECOPD, QMS remained statistically unchanged during the same period, with 36% of the patients exhibiting decreases in their QMS. Similar results have been found in hospitalised patients, where QMS decreased during the first 8 days of hospitalisation for AECOPD and only recovered at day 90.7 Thus, although both outcomes improved during an AECOPD, their timing of improvement differs, which may explain the positive correlation found between changes at T3-T1 between these two outcomes. Studies describing the pattern of QMS recovery in outpatients with AECOPD are needed to confirm these results and aid developing timely and personalised interventions.
Despite the novel findings in symptoms behaviour during AECOPD, this study has some limitations that need to be acknowledged. Treatment of exacerbations was not standardised, but rather prescribed according to the physician best judgment. Although the effects of therapies were not of interest in this study, it must be acknowledged that different combination of treatments might influence the recovery times and outcomes of individual patients. Characterisation of symptoms lack other important features, such as sputum purulence. These data can contribute to infer about the nature of the AECOPD (i.e., infective - viral and/or bacterial - or non-infective) and about the suitability of treatments prescribed. It is thus recommended to add sputum purulence to data collection in future study protocols.
In sum, this study showed that: i) dyspnoea is the most representative symptom at the onset of an AECOPD; ii) severity of cough is the first symptom to improve during the course of an AECOPD, and iii) changes in symptoms were correlated with FEV1 and QMS, which are predictors of COPD hospitalisations and mortality. Our findings evidence that timely management of symptoms is essential for patients’ recovery and should encourage health professionals to perform a comprehensive evaluation of outpatients with AECOPD using both patients reported symptoms and objective clinical outcome measures.
Conflict of interest statementThe authors declare no conflicts of interest.
This work was supported by Fundo Europeu de Desenvolvimento Regional (FEDER) through Programa Operacional Competitividade e Internacionalização (COMPETE) and Fundação para a Ciência e Tecnologia (FCT) under the projects UID/BIM/04501/2013, POCI-01-0145-FEDER-007628 and SFRH/BD/101951/2014.The authors would also like to acknowledge to Hélder Melro, Ana Machado and Sara Miranda for their assistance in data collection and to all patients and physicians from the Centro Hospitalar do Baixo Vouga for their collaboration in this study.