Pulmonary alveolar proteinosis (PAP) is a syndrome characterized by the accumulation of alveolar surfactant. Although auto-immune PAP is responsible for 90% of all cases, association with genetic abnormalities has also been established.1,2
The authors present the case of a 43 years old non-smoker female. She has a previous medical history of idiopathic CD4 lymphocytopenia, idiopathic segmental dystonia, lymphedema and septicaemia due to parvovirus B19. Two years ago, she was treated for Pneumocystis jiroveci pneumonia with cotrimoxazol with clinical resolution.
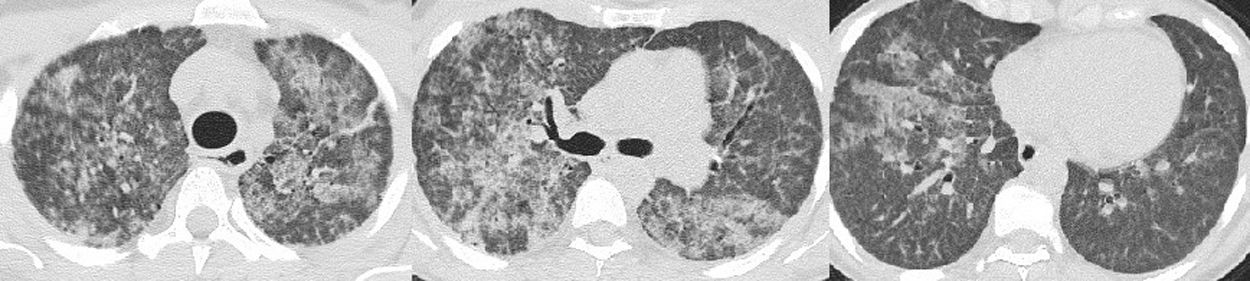
Ten months ago, she was admitted to Hospital for dyspnea with acute respiratory failure. She was assumed to have had acute cardiac failure with good response after diuretic treatment. After discharge, the echocardiogram was normal but in this context she performed a thoracic computed tomography scan which showed diffuse pulmonary ground-glass opacities and slight thickening of the interlobular septa — “crazy-paving” pattern (Fig. 1). At this time, she was referred to our Department of Thoracic Diseases due to these imaging findings and chronic respiratory insufficiency. She performed bronchoalveolar lavage that evidenced rare alveolar macrophages in an amorphous granular background with proteinaceous material; the cytopathological study was negative for malignant tumour cells and microbiological study showed no pathogenic microorganisms. Routine blood tests reported: hemoglobin 9.0 g/dL, platelets 59,000/µL, leukocytes 3100/µL, neutrophils 1181/µL, lymphocytes 1160/µL and monocytes 40/µL. Results were positive (1:320) for antinuclear antibodies (ANA), and negative for anti-neutrophil cytoplasmic antibodies (ANCA) and anti-extractable nuclear antigens (ENA) antibodies. Anti-granulocyte-macrophage colony-stimulating factor (anti-GM-CSF) antibodies were negative. A required genetic test for GATA-2 gene mutation identified a rs1573858 homozygote, benign variant. Although this mutation is considered not pathogenic, the whole clinical profile, except segmental dystonia, is typical of GATA-2 deficiency. Therefore, she was diagnosed with GATA-2 deficiency related PAP and whole lung lavage (WLL) was proposed. Right WLL was initially performed. A total of eight litres of warmed saline solution was instilled in the right lung and the fluid was then consecutively collected by gravity after opening an outflow tube. The procedure was aided by mechanical chest percussion. After 20 days, left WLL was performed with eight litres of saline solution (Fig. 2). There were no procedure complications and currently the patient does not require oxygen therapy.
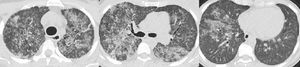
“Left whole lung lavage – procedure and fluid collected. (A) Patient is intubated with a double-lumen endotracheal tube in a right lateral decubitus position; 1 L of warmed saline is being instilled and subsequently collected by gravity after opening the outflow tube. Mechanical chest percussion is improving drainage. (B) From left to the right: from the 1 st to the 8th litre to be instilled and collected; the fluid becomes less opaque”.
Macrophage homeostasis is highly relevant to lung hygiene. Conditions reducing either the number or functions of alveolar macrophages would be expected to reduce their capacity to clear surfactant from the lung surface, promoting secondary PAP.1,3
GATA-2 belongs to a family of transcription factors that are critical regulators of gene expression in hematopoietic cells. Therefore, GATA-2 gene mutations may lead to haploinsufficiency associated with profound cytopenias. It remains unclear why but dendritic cells, monocytes, B and NK lymphoid cells are the ones mainly affected.3,4 Given this involvement, an association between GATA-2 insufficiency and PAP is to be expected. Given the usual abundance of alveolar macrophages in bronchoalveolar lavage fluid of these patients, PAP in GATA-2 deficiency must reflect more an alveolar macrophage dysfunction than a quantitative deficit, presumably by direct effects on alveolar macrophage phagocytosis.4 GATA-2 also interacts with different signaling cascades through modulating the expression of key receptors or transducing proteins, such as M-CSF receptor or phospholipase C.3Collin et al.3 reported the presence of 18% of PAP and 50% of abnormal pulmonary function cases in GATA-2 deficiency while Vinh et al.5 found it in 33% of patients. As expected, our patient had no detectable anti-GM-CSF antibodies. In our case, the mutation identified is considered a benign variant. However the clinical profile (except the segmental dystonia) is typically observed in GATA-2 deficiency and therefore another unidentified genetic hit may be hypothesized as a promoter factor.
The literature has highlighted the association between PAP and secondary opportunistic infections. However some studies have suggested that these microorganisms may even contribute to a secondary PAP.2,6 In our report, the patient presented a previous Pneumocystis jiroveci pneumonia. The authors question whether the potential role of Pneumocystis jiroveci is another contributing factor.
In recent years, some clinical syndromes have been associated with GATA-2 deficiency. Vinh et al. described in 2010 the Dendritic cell, Monocyte, B and NK Lymphoid Human Deficiency (DCML) Syndrome. This syndrome results from a progressive absence of multi-lymphoid or lymphoid-primed multipotent progenitors and a severe depletion of CD38+ granulocytic monocytic progenitors.5 In our report, the patient has a previous medical history of idiopathic CD4 lymphocytopenia. Considering these data, GATA-2 deficiency proved to be the explanation for the patient idiopathic CD4 lymphocytopenia. At the time of PAP diagnosis the patient also presented with a severe monocytopenia and a mild neutropenia. These findings are suggestive of DCML syndrome. However, a more detailed haematological study must be carried out to confirm this hypothesis. Ferreira et al. also described a patient with PAP that fulfilled the diagnostic criteria for DCML syndrome but until that date they had not confirmed the GATA-2 mutation.7
WLL is the current standard of care in auto-immune PAP.2 However, some secondary PAP cases have poor response to WLL and no reported series have specifically evaluated GATA-2 deficiency related PAP. In our report the patient presented an optimal clinical response. It will be important to note the clinical evolution in the coming months.
To sum up, our report shows a very rare cause of PAP suggesting that a benign variant of GATA-2 mutation may contribute to the onset of a complex syndrome. This emphasizes the need to search for secondary causes as well as the current available treatments.
Author contributionsVenerino Poletti and Stefano Maitan conceived the idea and made the diagnosis. Nuno China and Venerino Poletti collected the data and wrote the manuscript. Venerino Poletti and Carlo Gurioli were responsible for cytopathologic analysis. All the authors have read and approved the final version.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.