In Japan, Japanese cedar pollinosis (JCP) is one of the most prevalent forms of seasonal allergic rhinitis (AR).1 The JC pollen scattering period starts at the beginning of February and finishes at the end of April. JCP and asthma present concurrently; a previous survey showed that the prevalence of JCP in patients with asthma might be up to 30%–50%.2 Moreover, JCP is often associated with exacerbations of asthma.2 Therefore, JCP is a significant social problem in Japan. Pharmacotherapy for JCP includes histamine H1 receptor antagonist (HRA), leukotriene receptor antagonist (LTRA), and intranasal corticosteroid (INS).1 In addition, allergen-specific immunotherapy (ASI), including sublingual and subcutaneous immunotherapy, and antibody therapy, including omalizumab, are considered.1 ASI has been recognized as the only immunomodulatory therapy that can change the course of allergic disease by inducing allergen-specific immune tolerance, which may be associated with decreases in allergen-specific immunoglobulin E (sIgE) levels.1,3 Here we report five cases with JCP and asthma, whose sIgE levels against JC pollen are reduced by seasonal omalizumab treatment.
Five consecutive patients with asthma and JCP, who were followed up at Mitsubishi Kyoto Hospital and continuously treated with seasonal omalizumab for at least three consecutive years from December 2009 to May 2017, were retrospectively evaluated. Data regarding clinical characteristics, including age, body weight, height, smoking status, pulmonary function test, fractional exhaled nitric oxide, and blood test, were collected from medical records. The dose and dosing frequency of omalizumab was determined based on baseline total IgE (tIgE) and body weight according to dosing tables. We annually administered omalizumab between December and May. sIgE levels against JC pollen were determined using ImmunoCAP (Phadia, Tokyo, Japan). The lowest detection limit of sIgE level was 0.34UA/mL. tIgE and sIgE levels were analyzed at the start of each re-treatment with omalizumab.
All statistical analyses were performed using JMP® 10 statistical software package (SAS Institute; Cary, NC). A paired t-test was used to compare pre- and post-seasonal omalizumab treatment results. Data were expressed as mean±standard deviation. p values of <0.05 were considered statistically significant.
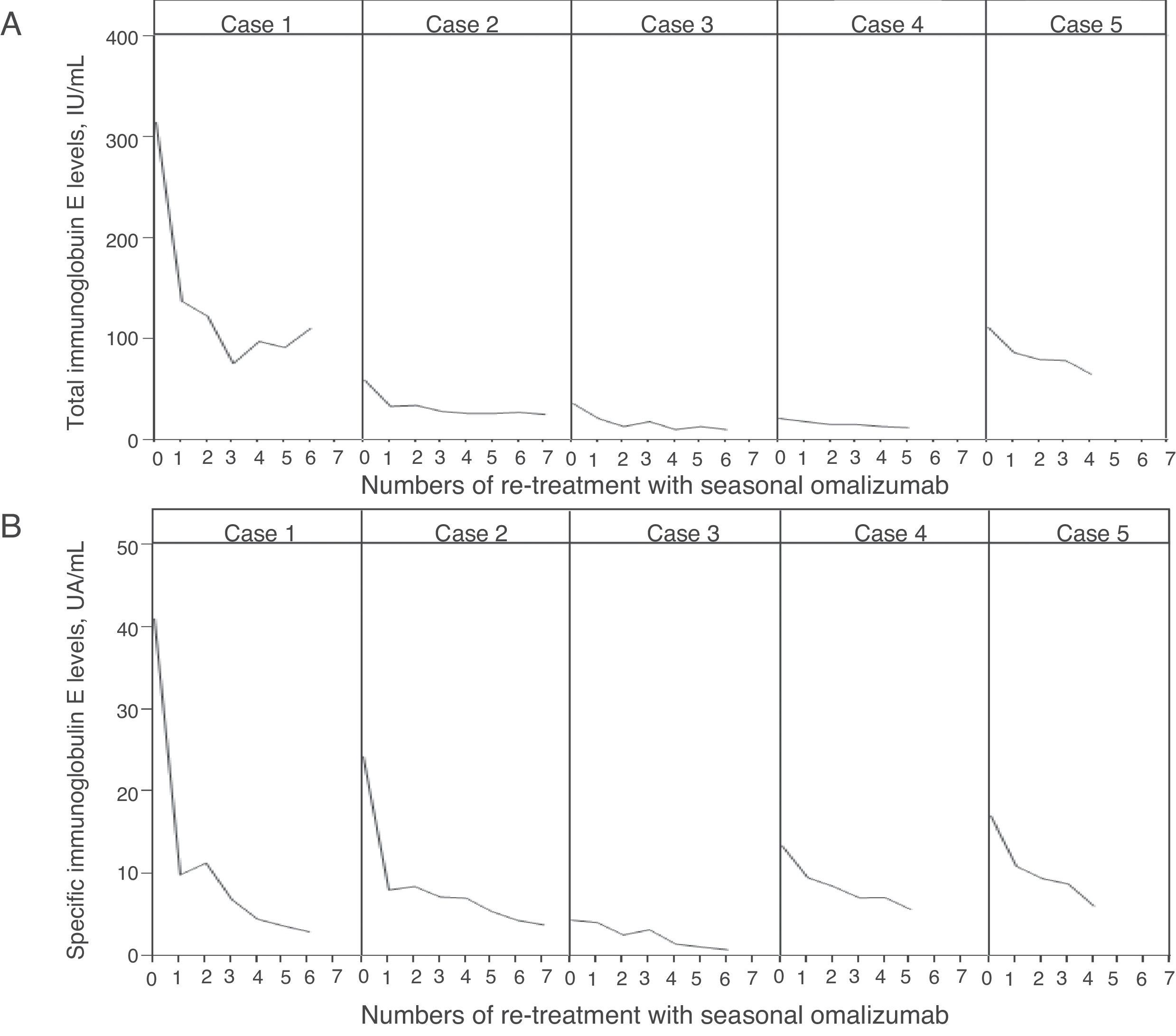
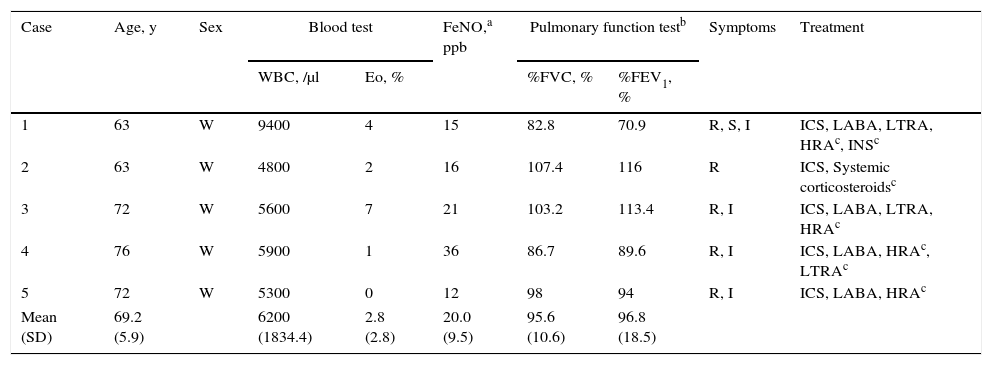
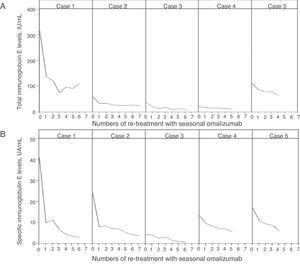
Baseline clinical characteristics of five patients are described in Table 1. All patients were non-smoking females. None of the patients had adverse effects to omalizumab. The changes in tIgE and sIgE levels after administration of seasonal omalizumab are described in Fig. 1. At the start of the fourth re-treatment with seasonal omalizumab, sIgE level had significantly decreased than at baseline (5.3±2.4UA/mL vs. 20.1±13.8UA/mL; p=0.04), whereas tIgE level had not changed compared with that at baseline (43.2±37.7IU/mL vs. 109.2±120.0IU/mL; p=0.08). In case 3 and 5, symptoms completely disappeared without additional treatment. In case 2, rhinorrhea could be controlled by additional treatment without systemic steroids but with HRA and LTRA. In case 1, we could stop additional treatment with persistence of minor sneezing and itchy eye. In case 4, LTRA could be discontinued with persistence of minor rhinorrhea.
Baseline clinical characteristics.
| Case | Age, y | Sex | Blood test | FeNO,a ppb | Pulmonary function testb | Symptoms | Treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| WBC, /μl | Eo, % | %FVC, % | %FEV1, % | ||||||
| 1 | 63 | W | 9400 | 4 | 15 | 82.8 | 70.9 | R, S, I | ICS, LABA, LTRA, HRAc, INSc |
| 2 | 63 | W | 4800 | 2 | 16 | 107.4 | 116 | R | ICS, Systemic corticosteroidsc |
| 3 | 72 | W | 5600 | 7 | 21 | 103.2 | 113.4 | R, I | ICS, LABA, LTRA, HRAc |
| 4 | 76 | W | 5900 | 1 | 36 | 86.7 | 89.6 | R, I | ICS, LABA, HRAc, LTRAc |
| 5 | 72 | W | 5300 | 0 | 12 | 98 | 94 | R, I | ICS, LABA, HRAc |
| Mean (SD) | 69.2 (5.9) | 6200 (1834.4) | 2.8 (2.8) | 20.0 (9.5) | 95.6 (10.6) | 96.8 (18.5) | |||
SD=standard deviation, WBC=white blood cell, Eo=eosinophil, FVC=forced vital capacity, FEV1=forced expiratory volume in 1s.
FeNO=fractional exhaled nitric oxide, R=rhinorrhea, S=sneezing, I=itchy eye, ICS=inhaled corticosteroid.
LABA=long-acting β2 stimulant, LTRA=leukotriene receptor antagonist, HRA=histamine H1 receptor antagonist, INS=intranasal steroid.
This report shows that repetitive seasonal omalizumab treatment reduces sIgE level against JC pollen in patients with JCP and mild asthma. Omalizumab is a recombinant humanized IgG1 monoclonal anti-IgE antibody, which binds IgE, rapidly suppressing free IgE concentrations.4 Furthermore, omalizumab may reduce IgE production through mechanisms of down-regulating low-affinity IgE receptor expression and IgE expressing lymphoblasts and memory cells.4 This phenomenon is observed with continuous omalizumab treatment. Therefore, this is the first report showing that repetitive seasonal omalizumab treatment can modulate sIgE synthesis. However, this report showed that tIgE levels did not change with repetitive seasonal omalizumab treatment. Ogino et al. reported that tIgE levels did not change at re-treatment with seasonal omalizumab.5 Therefore, future studies are required to evaluate the effect of seasonal omalizumab on sIgE level against pollens other than JC pollen.
Previous reports showed that re-treatment with seasonal omalizumab might be effective and safe in patients with seasonal AR.5,6 Here re-treatment with seasonal omalizumab for 5.6±1.1 times did not induce any adverse effects and reduced additional drugs required for managing JCP. Therefore, repetitive seasonal omalizumab treatment appears to be safe and effective. Clinical efficacy of omalizumab treatment in patients with seasonal AR has been shown to be associated with free IgE levels.7 This report showed that sIgE levels could be a predictive marker of the therapeutic response to seasonal omalizumab treatment. Therefore, future studies with a large number of participants are needed to evaluate the role of sIgE or free IgE levels as a predictive marker of the therapeutic response of seasonal omalizumab treatment.
ASI has been recognized as the only immunomodulatory therapy in AR. ASI-induced allergen-specific immune tolerance can be associated with decreases in allergen-sIgE levels.3 Therefore, seasonal omalizumab treatment has the potential for becoming another immunomodulatory therapy in seasonal AR and asthma.
Conflict of interestAll authors have no conflict of interest regarding to this report.