Asthma and obesity have a considerable impact on public health and their prevalence is increasing. Obesity is a known risk factor for asthma and can make it more difficult to control. Omalizumab is recommended in patients with severe allergic persistent asthma. The aim of this study was to assess the impact of omalizumab treatment in obese asthmatic patients with poorly controlled severe persistent asthma.
MethodologyA non-interventional, prospective study was conducted, in an outpatient asthma clinic. All patients with severe asthma who started treatment with omalizumab were included and followed over 12 months. The study population was divided into two groups (obese and non-obese) for statistical analysis (descriptive and comparative analysis).
ResultsThirty-two patients (19 obese) were followed. After 12 months of omalizumab treatment, there was a statistically significant improvement in body mass index, number of exacerbations in the previous year, rescue medication, disease control and lung function, in the whole population. At the end of the study obese patients had a significantly better lung function (FEV1) than non-obese.
DiscussionAs described in the literature, there was a significant reduction in the number of exacerbations in the previous year, rescue medication and better disease control, in the whole population. In relation to lung function, about which published data are inconsistent, treatment with omalizumab significantly improved it in obese patients.
ConclusionOur study showed that omalizumab significantly improved asthma control, reduced rescue medication and asthma exacerbations in all the population; and for the first time showed that obese patients achieved significantly improved lung function.
Asthma is a chronic heterogeneous disease, usually characterized by chronic airway inflammation, affecting almost 20% of the population in different countries.1 More than 15% of asthma patients may have severe asthma,2 and these patients are responsible for most of the health costs in asthma. According to International ERS/ATS guideline, severe asthma is defined as asthma that requires treatment with high dose inhaled corticosteroids plus a second controller (GINA steps 4–5) to prevent it from becoming ‘uncontrolled’ or asthma which remains ‘uncontrolled’ despite this therapy.3,4 Asthma is associated with a strong oxidative stress, which results both from the increase of the oxidative forces and from the reduction of the antioxidant capacity.5,6 The complexity of asthma is due to the multiple phenotypes and endotypes that might lead to the persistence of symptoms, the poor response to conventional pharmacotherapy and the susceptibility of the patient to comorbidities.5,7
Asthma2 and obesity6 have a considerable impact on public health and their prevalence has increased in recent years. Obesity is a known risk factor for asthma9–14 and for the severity of respiratory symptoms by increasing airway hyperreactivity and contributing towards a more difficult-to-control asthma phenotype.1,13–17 It is also known that obesity has a significant negative effect on all lung volumes, decreasing lung function.8
Obesity can make asthma more difficult to control10,14,17 and is associated with a lower quality of life10 and a reduced beneficial effect of asthma medications.10,11,13,14,17 Some of the known mechanisms for the increased risk of severe asthma in obese patients are: anatomical changes of the airways (such as obstruction),10 production of adipokines10,12,17 (such as leptin), impaired glucose-insulin metabolism,10,12,18 altered nutrient levels,10 genetic and epigenetic changes10 and inflammation.10,17 Systemic inflammation (particularly obese patients with high levels of IL-612,13 and TNF-α13), seems to have impact in severe asthma, but obese asthmatic patients do not appear to have increased airway cellular inflammation., they seem to have separate mechanisms that are specific to the obese state, rather than the conventional Th type 2-mediated inflammatory pathway.13,19 The current evidence suggests that while oxidative stress is important in asthma, it does not fully explain the characteristics associated with the obese-asthma phenotype.17
Omalizumab is a humanized monoclonal antibody that binds to the constant region (Fc) of immunoglobulin E (IgE). Omalizumab blocks the binding of IgE to its high affinity receptor Fc_R1, situated on the surface of the mast cells (and other cells). Consequently, activation of the mast cells and liberation of its pro-inflammatory mediators is inhibited.20 It is recommended as add-on therapy, in patients of six years or older, with severe allergic IgE-mediated persistent asthma, who are inadequately controlled despite the best available treatment (according to guidelines1) and need continuous or frequent treatment with oral corticosteroids (defined as 4 or more courses in the previous year).21
The literature about severe asthma in obese patients is scarce. Several clinical controlled trials showed that omalizumab reduces asthma-related symptoms, decreases corticosteroid use, and improves quality of life of asthmatic patients,22–24 however there is little information regarding the effects of omalizumab on obese patients with severe asthma. The aim of the current prospective study was to assess the impact of the treatment with omalizumab in obese patients with inadequately controlled severe persistent allergic (IgE-mediated) asthma.
MethodologyDesign and populationThis was a non-interventional, prospective study, conducted in an outpatient asthma clinic, from January 2014 to July 2017. All patients with severe asthma (diagnosed according to ERS/ATS guidelines3) who started treatment with omalizumab were included. Omalizumab treatment was approved by the therapeutic commission and hospital administration for all patients, as required in Portuguese hospitals. Minimal criteria for approval of omalizumab treatment for asthma were uncontrolled persistent severe allergic asthma, with frequent exacerbations.25 Data were collected in the beginning (T0), at each routine visit for omalizumab administration during 12 consecutive months and at the end (T12) of the study. Omalizumab was administered at 2 or 4 week intervals with doses based on serum IgE levels and body weight, as recommended.19 All subjects were informed about the study and written informed consent was obtained. The study was approved by the institutional ethics committee.
According to the body mass index (BMI), the study population was divided into two groups: obese patients (BMI≥30kg/m2) and non-obese patients (BMI<30kg/m2).
Variables analyzedBMI: the BMI is an accepted standard measure of overweight and obesity, which is a reflection of weight and height.26 Body weight and height of the subjects was evaluated on a SECA® anthropometric mechanical scale with stadiometer (SECA® – model 764, Porto, Portugal). During the study period the patients did not changed their lifestyle (physical activity or diet) in an orientated way, that is, they were not referred to the nutrition clinic or to gym. During the asthma assessment, at least 6 months prior to inclusion in this study, 8 patients (7 of whom were obese) were followed in our nutrition clinic (the remaining obese patients refused this consultation). In the nutritional clinic the nutritional status was evaluated, the energy and nutritional needs determined, an individualized diet plan prescription made and physical activity was promoted.
Exacerbations: all episodes of change from patient's usual status which required a change on patient's usual treatment were considered exacerbations. Exacerbations analysis was done by interview with the patient during each visit and through the digital clinical record analysis (admission to emergency room and unscheduled hospital or primary care visits).
ACT: at each visit the patient filled out the Asthma Control Test (ACT)27; asthma control was defined by ACT score above 19.
Spirometric parameters: all patients underwent forced spirometry (performed with a Vitalograph Pneumotrac™ Spirometer with Spirotrac® IV software), in accordance with ERS/ATS guidelines,28 using reference values published.29 The spirometry variables analyzed were forced expiratory volume during the first second (FEV1) and forced vital capacity (FVC). FEV1 is the most commonly used spirometric parameter in the evaluation of asthmatic patients lung function1 and FVC is notably decreased in obese people.8 These two spirometric parameters were evaluated simultaneously in order to analyze if obesity had influence on both in the same way.
Equivalent dose of budesonide: the equivalent dose of budesonide was calculated according to the prescriptions in medical visits and was expressed in equivalent dose of budesonide.
Statistical analysisA descriptive analysis of the study population followed by a comparative analysis between the two study groups was made and then an analysis of each of the groups. Statistical analysis was performed using IBM SPSS Statistics version 21 (2012 SPSS Inc., IBM Company, Chicago, US). Categorical and continuous variables were analyzed using descriptive statistics as appropriate. Statistical analysis was performed using Student's t-test for independent samples and Student's t-test for paired samples. A p-value of <0.05 was considered statistically significant.
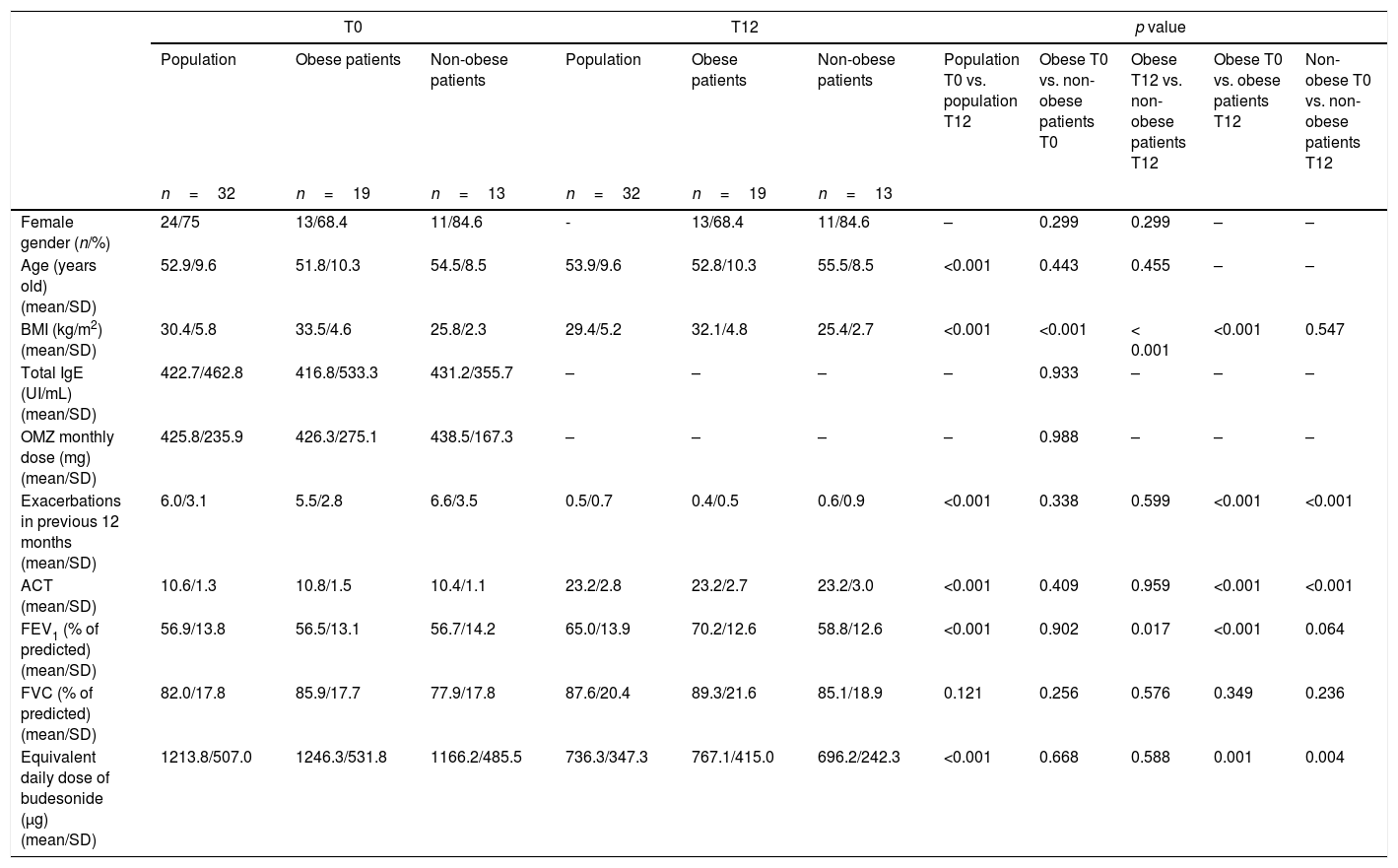
ResultsA total of 32 adults with severe asthma were prospectively followed during a 12 month period. Most of the patients were female (75%). The mean age was 53 years-old. Nineteen (59.4%) patients were obese. Table 1 lists the characteristics of the study population and of each group (obese and non-obese), at T0 and T12 “show Table 1 here”. At the beginning of the study there were no statistically significant differences between the 2 groups regarding gender, age, total IgE, omalizumab monthly dose, exacerbations in the previous 12 months, ACT, FEV1, FVC and equivalent daily dose of budesonide, with the exception of BMI which was significantly higher in obese patients group (33.5 vs. 25.8kg/m2, p<0.001).
Characteristics of the study population, obese patients and non-obese patients, at T0 and T12; and comparative analyses of population, between groups and each group, at T0 and T12.
| T0 | T12 | p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Obese patients | Non-obese patients | Population | Obese patients | Non-obese patients | Population T0 vs. population T12 | Obese T0 vs. non-obese patients T0 | Obese T12 vs. non-obese patients T12 | Obese T0 vs. obese patients T12 | Non-obese T0 vs. non-obese patients T12 | |
| n=32 | n=19 | n=13 | n=32 | n=19 | n=13 | ||||||
| Female gender (n/%) | 24/75 | 13/68.4 | 11/84.6 | - | 13/68.4 | 11/84.6 | – | 0.299 | 0.299 | – | – |
| Age (years old) (mean/SD) | 52.9/9.6 | 51.8/10.3 | 54.5/8.5 | 53.9/9.6 | 52.8/10.3 | 55.5/8.5 | <0.001 | 0.443 | 0.455 | – | – |
| BMI (kg/m2) (mean/SD) | 30.4/5.8 | 33.5/4.6 | 25.8/2.3 | 29.4/5.2 | 32.1/4.8 | 25.4/2.7 | <0.001 | <0.001 | < 0.001 | <0.001 | 0.547 |
| Total IgE (UI/mL) (mean/SD) | 422.7/462.8 | 416.8/533.3 | 431.2/355.7 | – | – | – | – | 0.933 | – | – | – |
| OMZ monthly dose (mg) (mean/SD) | 425.8/235.9 | 426.3/275.1 | 438.5/167.3 | – | – | – | – | 0.988 | – | – | – |
| Exacerbations in previous 12 months (mean/SD) | 6.0/3.1 | 5.5/2.8 | 6.6/3.5 | 0.5/0.7 | 0.4/0.5 | 0.6/0.9 | <0.001 | 0.338 | 0.599 | <0.001 | <0.001 |
| ACT (mean/SD) | 10.6/1.3 | 10.8/1.5 | 10.4/1.1 | 23.2/2.8 | 23.2/2.7 | 23.2/3.0 | <0.001 | 0.409 | 0.959 | <0.001 | <0.001 |
| FEV1 (% of predicted) (mean/SD) | 56.9/13.8 | 56.5/13.1 | 56.7/14.2 | 65.0/13.9 | 70.2/12.6 | 58.8/12.6 | <0.001 | 0.902 | 0.017 | <0.001 | 0.064 |
| FVC (% of predicted) (mean/SD) | 82.0/17.8 | 85.9/17.7 | 77.9/17.8 | 87.6/20.4 | 89.3/21.6 | 85.1/18.9 | 0.121 | 0.256 | 0.576 | 0.349 | 0.236 |
| Equivalent daily dose of budesonide (μg) (mean/SD) | 1213.8/507.0 | 1246.3/531.8 | 1166.2/485.5 | 736.3/347.3 | 767.1/415.0 | 696.2/242.3 | <0.001 | 0.668 | 0.588 | 0.001 | 0.004 |
ACT: asthma control test; BMI: body mass index; FEV1: forced expiratory volume in first second; FVC: forced vital capacity; IgE: immunoglobulin E; OMZ: omalizumab; SD: standard deviation.
The comparative analysis of the population at T0 and T12 showed that, after 12 months of treatment with omalizumab, there was a statistically significant improvement in all the parameters under analysis, with exception of FVC. There was a significant reduction in BMI (30.4 vs. 29.4kg/m2, p<0.001), in the number of exacerbations in the previous 12 months (6.0 vs. 0.5, p<0.001) and in the equivalent daily dose of budesonide (1213.8 vs. 736.3μg, p<0.001); and a significant improvement in ACT (10.6 vs. 23.2, p<0.001) and in FEV1 (56.9 vs. 65.0%, p<0.001) (Table 1).
The comparative analysis of the two groups showed that at the end of the study, obese patients had a significantly higher FEV1 than non-obese patients (70.2 vs.58.8%, p=0.017). There were no other significant differences between the two groups, unless BMI which was significantly higher in obese patients (32.1 vs. 25.4kg/m2, p<0.001) (Table 1).
The analysis of each group showed that: obese patients had a statistically significant improvement in ACT and FEV1, and a statistically significant reduction in BMI, exacerbations in the previous 12 months and equivalent daily dose of budesonide; non-obese patients had a statistically significant improvement in ACT and a significant reduction in the exacerbations in the previous 12 months and in the equivalent daily dose of budesonide (Table 1). In the end of the study 18 (94.7%) obese patients had a loss of 4.9±3.9% of their initial weight.
DiscussionIn the present study, the majority of the population was obese. The risk factor obesity for the development of asthma is widely described in the literature. A meta-analysis based on prospective studies of Bather and Sutherland showed that the odds of developing asthma for individuals with obesity compared to those with normal weight were twice as high (OR 1.92, 95% CI 1.43–2.59).30,31 In addition to being a risk factor for asthma, obesity seemed to promote a more persistent and severe asthma phenotype, independently of socio-demographic and lifestyle determinants, such as physical activity and dietary patterns.13,14,16,17,30
In patients with severe allergic IgE-mediated persistent asthma it had already been demonstrated that treatment with add-on omalizumab significantly reduced symptoms,32,33 rescue medication32,33 and asthma exacerbations.20,32,33 In our study, the comparative analysis of the population at T0 and T12 showed that, after 12 months of treatment with omalizumab, there was a statistically significant improvement in almost all the analyzed parameters. As described, there was a significant reduction in the number of exacerbations in the previous 12 months, in the equivalent daily dose of budesonide, and a significant improvement in ACT. In the analysis performed for each group separately (obese and non-obese), similarly to what happened with the population, there was a significant improvement in the three parameters already described.
The data about the effect of omalizumab on lung function are inconsistent.33 The study of Özgür et al. showed that long-term therapy with omalizumab had a significant improvement in lung function (FEV1).34 In the present study, after 12 months of treatment with omalizumab, not only there was a significant improvement in FEV1 in the study population, but there was also a statistically significant difference between the two study groups regarding lung function. At the beginning of the study, the two groups were homogeneous, with no statistically significant differences in any of the parameters under analysis (gender, age, total IgE, omalizumab dose, number of exacerbations in the previous 12 months, ACT, FEV1, FVC and equivalent dose of busonide), with the exception of BMI. At the end of the study, the stability between groups was maintained, with the exception of FEV1 which was different for the two groups, as obese patients had a significantly higher FEV1 compared to non-obese patients, without significant changes in FVC. The analysis of each study group in T0 vs. T12, showed that FEV1 of obese patients was significantly higher in T12, and that there was no statistically significant difference in FEV1 of non-obese patients. Based on these results we believe that the significant improvement of lung function in the study population was mainly due to the improvement observed in obese patients group, because in non-obese patients there was no significant difference. In addition, we found that the improvement in lung function was most probably not due to weight loss (which in obese patients was not satisfactory; it was below 5%35), because unlike FEV1, there was no significant change in FVC (which is the spirometric parameter most influenced by obesity8).
The mechanisms underlying the clinical and anti-inflammatory efficacy of omalizumab are not fully understood. The published data about the impact of omalizumab in lung function of asthmatic patients are inconsistent and this may be due to the fact that there are no studies specifically with obese patients, which as we have now seen, was the subpopulation on which there was greater impact. Omalizumab can neutralize free IgE, interrupting a key step in the allergic inflammatory cascade, preventing IgE from binding to mast cells, basophils, and dendritic cells, and down-regulating IgE receptor expression in these inflammatory cells, thereby inhibiting degranulation and the release of inflammatory mediators.36 Anti-IgE therapy is a treatment modality that mediates its effects through decreasing inflammation following improved anti-oxidant capability,35 this loss of inflammation may have been the mechanism responsible for the lung function improvement seen in our obese asthmatic patients.
The mechanisms underlying the epidemiology of obesity and asthma phenotypes are still not clear. It is known that the increased abdominal and chest wall mass causes a decrease in the functional residual capacity and a reduction in lung and tidal volumes16 and, that obesity is a pro-inflammatory state leading to an increase in inflammation.16 Weight loss is associated with improvement in lung function, symptoms, morbidity and overall health status,13,16 and it should be part of asthma management in obese patients.10 In this study, there was a reduction in BMI after 12 months of treatment with omalizumab, which was significantly reduced in the whole population and in the obese patients group. According to our data the reduction in BMI may have been due to decreased use of corticosteroids and to less limitation in the activities of daily living as a consequence of the best asthma control and FEV1 improvement, since no measures were taken towards weight loss during the 12-month follow-up.
This study was limited by the small sample size and also because the particular measures adopted by each patient for weight loss were not studied, however it was a prospective study where, for the first time, the subpopulation of obese patients with severe asthma was studied. Despite the limitations, with this study we found that the impact of treatment with omalizumab is greater in obese patients. Although weight loss is important in obese patients with asthma and, in the present study the weight loss was significant in the group of patients with improvement in almost all the parameters analyzed (obese patients group), we could not explain this relationship. It might have been the better disease control which consequently led to less physical inactivity and hence weight loss or there may have been another mechanism behind this relationship. Recent studies suggest that obesity may have a causal relationship with asthma,37 but more prospective studies are still needed in the subpopulation of obese patients with severe asthma.
ConclusionIn summary, our study showed that omalizumab, used as an add-on to optimized standard therapy, in patients with severe allergic IgE-mediated persistent asthma, significantly improved asthma control, reduced rescue medication and asthma exacerbations. We have demonstrated for the first time that, in the subpopulation of obese patients with severe asthma, treatment with omalizumab significantly improved lung function (FEV1). Therefore, our results reinforced the importance of omalizumab used as add-on therapy especially in obese severe asthmatic patients where it has a greater impact.
Our study also provides a rational for future studies exploring the obese-asthmatic patients treated with omalizumab and the immunological pathways that might lead to asthma that is associated with obesity.
Conflicts of interestThe authors have no conflicts of interest to declare.