Spirometry is the most frequently used test to evaluate the progression of lung damage in cystic fibrosis (CF). However, there has been low sensitivity in detecting early lung changes. In this context, our objective was to identify the correlation between parameters of volumetric capnography (VCap) and spirometric parameters during a submaximal treadmill exercise test.
MethodsA cross-sectional and controlled study which included 64 patients with CF (CFG) and 64 healthy control subjects (CG) was performed. The CFG was from a university hospital and the CG from local schools. All participants underwent spirometry and VCap before, during and after the submaximal treadmill exercise test. The main variable analyzed by VCap was the slope of phase 3 (slope 3), which indicates the [exhaled carbon dioxide] at the end of expiration, and expresses the heterogeneity of gas emptying in pulmonary periphery. The correlation analysis between spirometry and VCap was conducted using the Spearman correlation test, considering α=0.05.
ResultsThe indices analyzed by VCap showed correlation with parameters of VCap. Slope 3 showed an inverse correlation with forced expiratory volume in the first second of forced vital capacity (FEV1) in both groups and at all moments of the submaximal treadmill exercise test. Forced vital capacity (FVC) and FEV1/FVC ratio showed an inverse correlation with slope 3 only for CFG. Values of slope 3 corrected by the spontaneous tidal volume (VT) and end-tidal carbon dioxide tension (PetCO2) showed results similar to slope 3 analyzed separately.
ConclusionParameters of VCap such as slope 3, slope 3/VT and slope 3/PetCO2 correlated with sensitive variables of spirometry such as FEV1, FVC and FEV1/FVC ratio. For the evaluated variables, there was consistency in the correlation between the two tests, which may indicate the impact of CF on pulmonary physiology.
Patients with cystic fibrosis (CF) (OMIM: #219700) from birth, show a progressive deterioration of lung structures,1,2 with functional changes that are perpetuated throughout life. These include decreased: peripheral capillary oxygen saturation (SpO2), the ratio of anatomic dead space and expired tidal volume (VD/VT), forced expiratory volume in one second (FEV1) and the ability to perform exercise. Moreover, increased respiratory rate (RR), heart rate (HR) and partial pressure of exhaled carbon dioxide (PetCO2) in arterial blood have been shown.2–4
Challenges in the management of lung disease in CF include the early detection of lung damage, monitoring of disease progression and the identification and validation of parameters to evaluate response to treatment.5–7 In this context, studies have proposed the use of new markers and instruments, such as clinical scores,8 computed tomography,6 sputum culture or endotracheal secretion,9 lung clearance index,10 functional tests [six-minute walk test (6-MWT)11 and treadmill tests4], transepithelial potential difference,12 exhaled nitric oxide,13 impulse oscillometry,14 condensed exhaled15 and volumetric capnography (VCap),4,5,7,16 with the latter recently being considered a promising useful tool in the assessment of functional impairment of the lungs.4,5,7,16 Also, until now, spirometry has been considered the best lung function test to monitor lung disease progression in CF for more than two decades, but the VCap has emerged as a feasible new tool.5–7,16
The VCap may reflect the increased alveolar heterogeneity with the evolution of lung disease.4,5,7,16 The VCap presents a curve from the measurement of the concentration of end tidal CO2, compared to values measured simultaneously in expired volumes during a single respiratory cycle, representing an alternative in the search for links between structural damage and functional changes.5,16 VCap is a noninvasive, easy to use method, with simple maintenance and low cost.4 Also, recently, alterations of the small airways in patients with CF and normal spirometry were detected by VCap showing the feasibly of performing this test early, before severe pulmonary structure changes occur.5
The VCap allows calculations of several indices among them the slope of phase 3 (slope 3) and little is known about its behavior during the physical exercise in individuals with CF. Slope 3 is a measure of the gas elimination curve and contains information about the transport of gases in the alveolar airways of the pulmonary periphery, hence the alveolar plateau. In addition, the slope of phase 2 (slope 2) describes the removal of CO2 from the alveoli, representing the mixture of alveolar gas and VD gas. Thus, slope 2 is determined by the abrupt rise of CO2 in the exhaled air from the VD.17 Thus, the VCap reflects disturbances of lung function and may be useful as a non-invasive method of estimating the change in function in many lung diseases.4,5,7,16
The combination of VCap and exercise and the comparison with an already validated technique, such as spirometry, can provide us with valuable information about gas exchange during exercise, as well as, help us understand the limitation of these individuals in activities of daily life.
The scientific community has turned its attention to methods and parameters that may be more sensitive in the early detection of these kinds of lung damage so that they can be combated early. Measurements of gas exchange of various forms during rest have already been obtained. However, the role of physical exercise, as an indicator of prognosis and/or therapeutic instrument, is an area of research interest in several diseases, particularly respiratory diseases. Physical exercise in CF has been studied as a marker of prognosis or as a tool to improve quality of life, and respiratory morbidity.1,3,4
In this context, the objective of the study was to verify the correlation between VCap parameters during submaximal treadmill exercise test with validated markers of spirometry in patients with CF and healthy controls.
Material and methodsWe conducted a cross-sectional and controlled study, which included patients with CF (CFG) from a university hospital and healthy volunteers as a control group (CG). All patients with CF who agreed to participate were included. Those over 18 years of age, and caregivers of minors, signed the consent form, and the study followed the ethical standards of the Helsinki agreement and was approved by the Ethics Committee of the institution (#1182/2009).
Group of patients with cystic fibrosis (CFG)Inclusion criteria was age between six and 25 years (inclusive). The CF was diagnosed according to international consensus criteria for sweat test (chloride values above 60mEq/L) and/or the presence of two CFTR mutations identified (cystic fibrosis transmembrane regulator).18
The clinical stability was controlled by the use of two clinical scores: Cystic Fibrosis Foundation Clinical Score and Cystic Fibrosis Clinical Score8,19,20 and the severity of the disease was classified per the Shwachman–Kulczycki score.21 Patients with lung exacerbation were not included. Also, we did not include patients on oxygen therapy. All patients with CF performed the submaximal treadmill exercise test without interruption.
Control group (CG)The CG included children, adolescents and young healthy adults aged between six to 25 years, randomly selected among students from public and private schools of the same university district. All participants answered a questionnaire about health and none had acute respiratory disease at the time of data collection by clinical evaluation and spirometry markers.
Evaluation proceduresAll participants performed sequentially.
SpirometryBefore starting the submaximal treadmill exercise test, the follow anthropometric measurements were conducted: weight (kg), height (cm) and calculation of body mass index (BMI). Next, all subjects underwent spirometry according to the guidelines of the American Thoracic Society.22,23 Spirometry was performed using a CPFS/D spirometer (MedGraphics, Saint Paul, Minnesota, USA; PF BREEZE Software version 3.8 B for Windows 95/98/NT), and the percentage of predicted values of FEV1 (FEV1% predicted), forced vital capacity (FVC% predicted) and the ratio of FEV1/FVC were analyzed. Spirometry data are shown in percentage of the predicted value according to the Polgar and Promadhat (1971), Pereira et al. (2007) and Duarte et al. (2007) equations.23–25
Volumetric capnography (VCap)For VCap, a Co2smo Plus® DX 8100 Monitor (Novametrix Medical Systems, Wallingford, CT, USA) was used, which monitored the participants during the submaximal treadmill exercise test. This is a noninvasive monitor with capnography, pulse oximetry and pneumotachography function. The capnography and pneumotachography measures were obtained in real time by analysis of expired gases. The Co2smo Plus® was connected to a computer equipped with software to record the flow, volume, pressure, pressure-volume, flow-volume and capnography measurements and curves.
The sensor monitor was connected to a mouthpiece, and a nose clip was used to prevent air escaping. At the end of the collection, an offline sequence of subjects’ respiratory cycles was selected for admission to a coefficient of variation for the lowest expired VT of 25% of the current volume of the mean, and the PetCO2 was admitted to a coefficient of variation less than 10% in millimeters of mercury (mmHg). Respiratory cycles presenting a value of zero in slope 3 were eliminated. The result obtained was the average of the variables during four minutes of monitoring.4,5
The following measures were analyzed:
- i.
Slope 3 was calculated using a linear regression curve of CO2/exhaled volume, measured in millimeters of mercury per liter (mmHg/L).
- ii.
VT: volume of air exhaled in one breath, measured in milliliters (mL).
- iii.
Slope 2 was calculated using a linear regression curve of CO2/exhaled volume, measured in mmHg/L.
- iv.
RR: measured in breaths per minute.
- v.
Volume carbon dioxide (VCO2): volume of carbon dioxide exhaled in one breath in mL.
- vi.
PetCO2, measured in mmHg.
- vii.
VD: volume of air that remains in the conducting airways, measured in mL.
- viii.
Alveolar tidal volume (VTalv): VT minus the VD in mL.
- ix.
VD/VT ratio.
As the Co2smo Plus® monitor has pulse oximetry capability we also collected: (i) HR measured in beats per minute and (ii) SpO2 measured as a percentage (%).4,5
Submaximal treadmill exercise testTo determine how these variables are affected by exercise and lung disease, the participants walked on a Caloi Electronics® Pro CL 5004 treadmill for a period of six minutes; with increasing velocity, according to the tolerance of each individual. In this context, aiming at a submaximal test, the participants did not reach full capacity of exercise, choosing the proper exercise intensity at a submaximal level of effort.4,26,27
Just before the start of the submaximal treadmill exercise test, the participant remained at rest for five minutes for the measurement of cardiorespiratory variables, VCap and modified Borg scale (the classification of the subjective perception of effort). Then the submaximal treadmill exercise test began and cardiorespiratory data (RR, HR, SpO2 and VCap) were measured throughout the submaximal treadmill exercise test by the Co2smo Plus®.4
The exercise was accompanied by verbal stimulus (“you are doing very well; can you go a little faster?”) in the first minute and at the end of each minute. Throughout the activity participants used a nozzle connected to the capnography sensor.
The exercise was based on the 6-MWT and protocols developed for treadmills.4,27–31
The modified Borg scale is an instrument that evaluates the subjective perception of the effort measured by a questionnaire, 0 being no fatigue, up to the value of 10 that indicates exhaustive exhaustion. The scale was applied at rest (before) and immediately after the submaximal exertion test. The Borg scale was applied, only giving the explanation of the association with the perception of effort and that higher numbers represented a worst status. The Borg scale is shown in Supplementary Material 1.
Statistical analysisStatistical analysis was performed for the CG and CFG at five different times in relation to the exercise: (1) basal; (2) one and two minutes into the test; (3) three and four minutes into the test; (4) five and six minutes into the test and (5) immediately post exercise. To calculate the values from the markers achieved in VCap we used the mean from all the data obtained during the period of the analysis (five different periods of time). We also include the cardiorespiratory adaptation period to promote a complete understanding of the response to two tools used in the submaximal treadmill exercise test performed. The association of the FVC, FEV1 and FEV1/FVC between patients with CF and healthy control subjects was performed by the Mann–Whitney test. Data were processed by the Statistical Package for Social Sciences software version 21.0 (SPSS Inc., Chicago, IL, USA). Correlations between spirometry and VCap were assessed by Spearman correlation test. In the data, the value of the Rho correlation coefficient is indicated. In all analyses, we adopted an alpha value of 0.05.
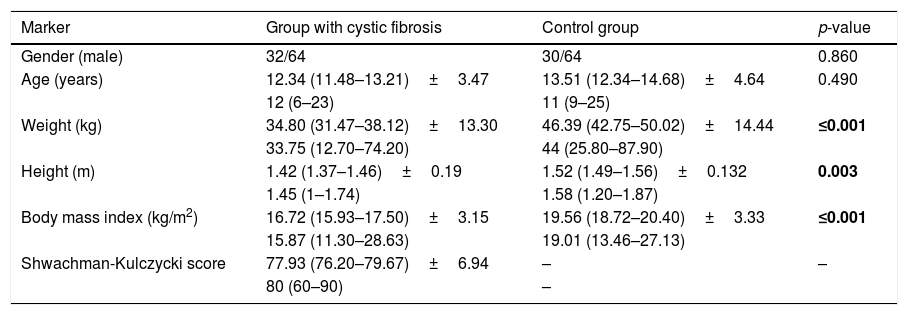
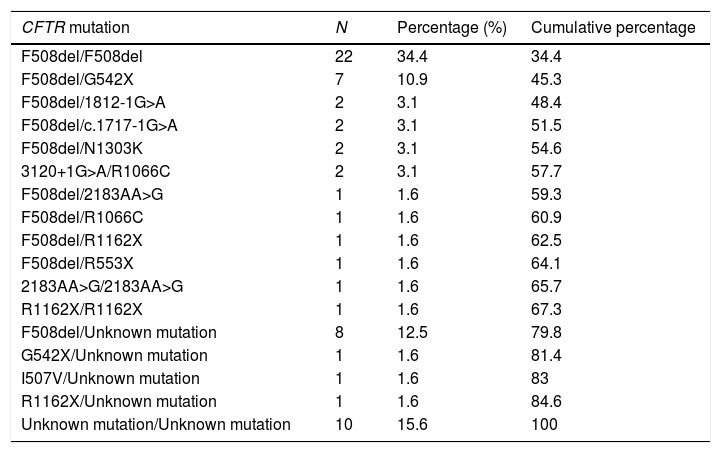
ResultsA total of 128 subjects were evaluated, 64 in each group. There was no statistical difference in age and gender between the groups. Anthropometric variables were lower in CFG for weight, height and BMI compared to CG (Table 1). Among CFG, the Shwachman–Kulczycki score ranged from 60 to 90 (77.93±6.94) (Table 1) and the profile of the CFTR mutations identified is shown in Table 2. In the modified Borg scale, subjects showed higher values after submaximal treadmill exercise test. The greatest sensation of dyspnea occurred in the CFG (p-value<0.05).
Distribution of patients with cystic fibrosis and healthy controls included in the study according to the clinical and laboratory markers.
| Marker | Group with cystic fibrosis | Control group | p-value |
|---|---|---|---|
| Gender (male) | 32/64 | 30/64 | 0.860 |
| Age (years) | 12.34 (11.48–13.21)±3.47 | 13.51 (12.34–14.68)±4.64 | 0.490 |
| 12 (6–23) | 11 (9–25) | ||
| Weight (kg) | 34.80 (31.47–38.12)±13.30 | 46.39 (42.75–50.02)±14.44 | ≤0.001 |
| 33.75 (12.70–74.20) | 44 (25.80–87.90) | ||
| Height (m) | 1.42 (1.37–1.46)±0.19 | 1.52 (1.49–1.56)±0.132 | 0.003 |
| 1.45 (1–1.74) | 1.58 (1.20–1.87) | ||
| Body mass index (kg/m2) | 16.72 (15.93–17.50)±3.15 | 19.56 (18.72–20.40)±3.33 | ≤0.001 |
| 15.87 (11.30–28.63) | 19.01 (13.46–27.13) | ||
| Shwachman-Kulczycki score | 77.93 (76.20–79.67)±6.94 | – | – |
| 80 (60–90) | – |
kg, kilogram; m, meter. For variables with numeric distribution, the data are described as mean (confidence interval)±standard deviation; median (minimum to maximum). The difference between groups was calculated by the χ2 test for categorical variables and by Mann–Whitney test for numerical data considering α=0.05. Positive p-values are shown in bold.
CFTR mutations genotypes of patients with cystic fibrosis enrolled in the study (n=64).
| CFTR mutation | N | Percentage (%) | Cumulative percentage |
|---|---|---|---|
| F508del/F508del | 22 | 34.4 | 34.4 |
| F508del/G542X | 7 | 10.9 | 45.3 |
| F508del/1812-1G>A | 2 | 3.1 | 48.4 |
| F508del/c.1717-1G>A | 2 | 3.1 | 51.5 |
| F508del/N1303K | 2 | 3.1 | 54.6 |
| 3120+1G>A/R1066C | 2 | 3.1 | 57.7 |
| F508del/2183AA>G | 1 | 1.6 | 59.3 |
| F508del/R1066C | 1 | 1.6 | 60.9 |
| F508del/R1162X | 1 | 1.6 | 62.5 |
| F508del/R553X | 1 | 1.6 | 64.1 |
| 2183AA>G/2183AA>G | 1 | 1.6 | 65.7 |
| R1162X/R1162X | 1 | 1.6 | 67.3 |
| F508del/Unknown mutation | 8 | 12.5 | 79.8 |
| G542X/Unknown mutation | 1 | 1.6 | 81.4 |
| I507V/Unknown mutation | 1 | 1.6 | 83 |
| R1162X/Unknown mutation | 1 | 1.6 | 84.6 |
| Unknown mutation/Unknown mutation | 10 | 15.6 | 100 |
CFTR, cystic fibrosis transmembrane regulator; N, number of patients.
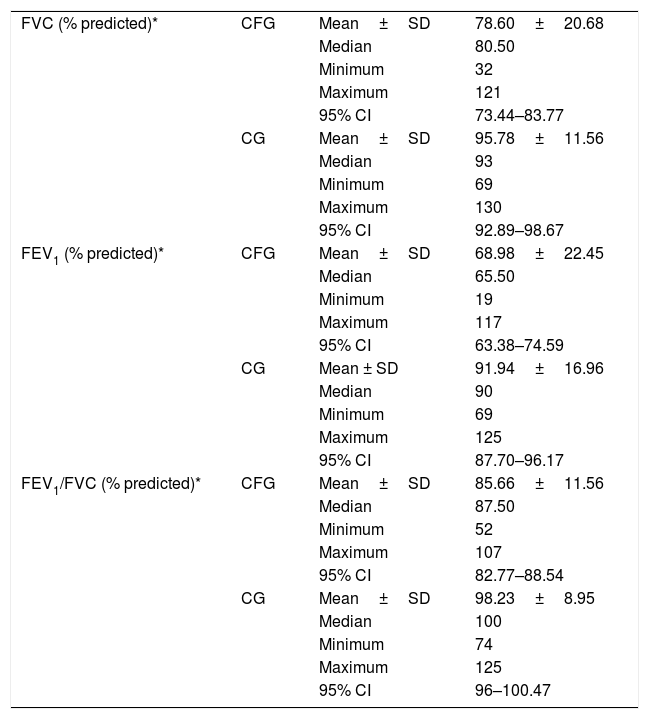
CFG had lower spirometric variables, as a percentage of the predicted value, compared to CG (FVC%: 78.61±20.68 versus 95.78±11.56; FEV1%: 68.98±22.45 versus 91.94±16.96 and FEV1/FVC%: 85.66±11.56 versus 98.23±8.95) (Table 3) (p-value<0.05).
Descriptive analysis of spirometry variables considering the groups with cystic fibrosis (CFG) and control (CG).
| FVC (% predicted)* | CFG | Mean±SD | 78.60±20.68 |
| Median | 80.50 | ||
| Minimum | 32 | ||
| Maximum | 121 | ||
| 95% CI | 73.44–83.77 | ||
| CG | Mean±SD | 95.78±11.56 | |
| Median | 93 | ||
| Minimum | 69 | ||
| Maximum | 130 | ||
| 95% CI | 92.89–98.67 | ||
| FEV1 (% predicted)* | CFG | Mean±SD | 68.98±22.45 |
| Median | 65.50 | ||
| Minimum | 19 | ||
| Maximum | 117 | ||
| 95% CI | 63.38–74.59 | ||
| CG | Mean ± SD | 91.94±16.96 | |
| Median | 90 | ||
| Minimum | 69 | ||
| Maximum | 125 | ||
| 95% CI | 87.70–96.17 | ||
| FEV1/FVC (% predicted)* | CFG | Mean±SD | 85.66±11.56 |
| Median | 87.50 | ||
| Minimum | 52 | ||
| Maximum | 107 | ||
| 95% CI | 82.77–88.54 | ||
| CG | Mean±SD | 98.23±8.95 | |
| Median | 100 | ||
| Minimum | 74 | ||
| Maximum | 125 | ||
| 95% CI | 96–100.47 |
FVC (% predicted), forced vital capacity; FEV1 (% predicted), forced expiratory volume in one second; CFG, group with cystic fibrosis; CG, control group. *, p-value<0.001. The statistical analysis was performed regarding the Mann–Whitney test. α=0.05.
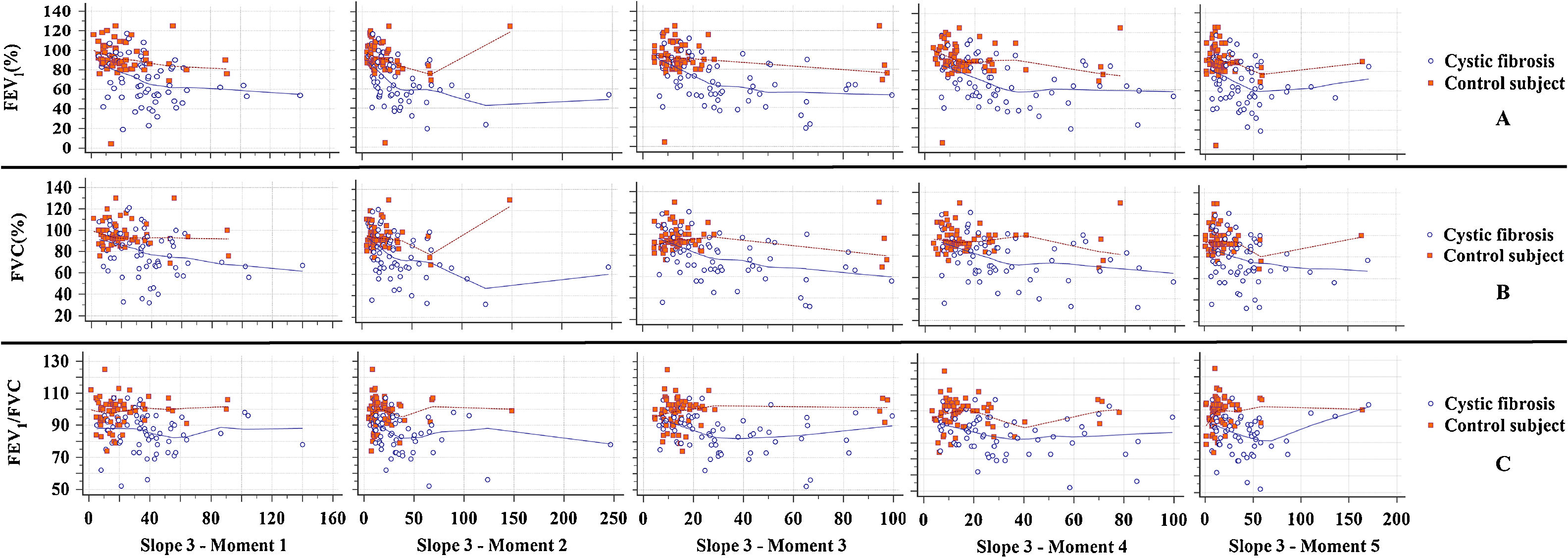
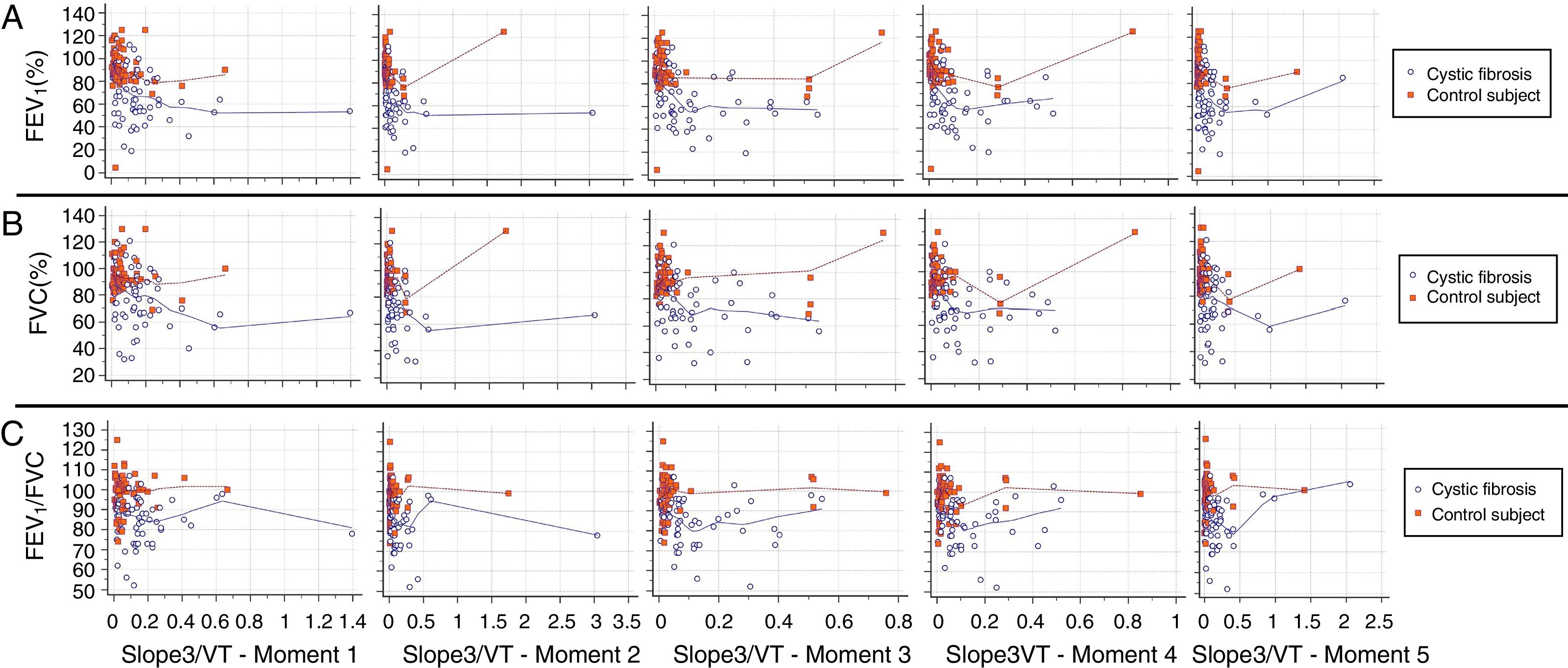
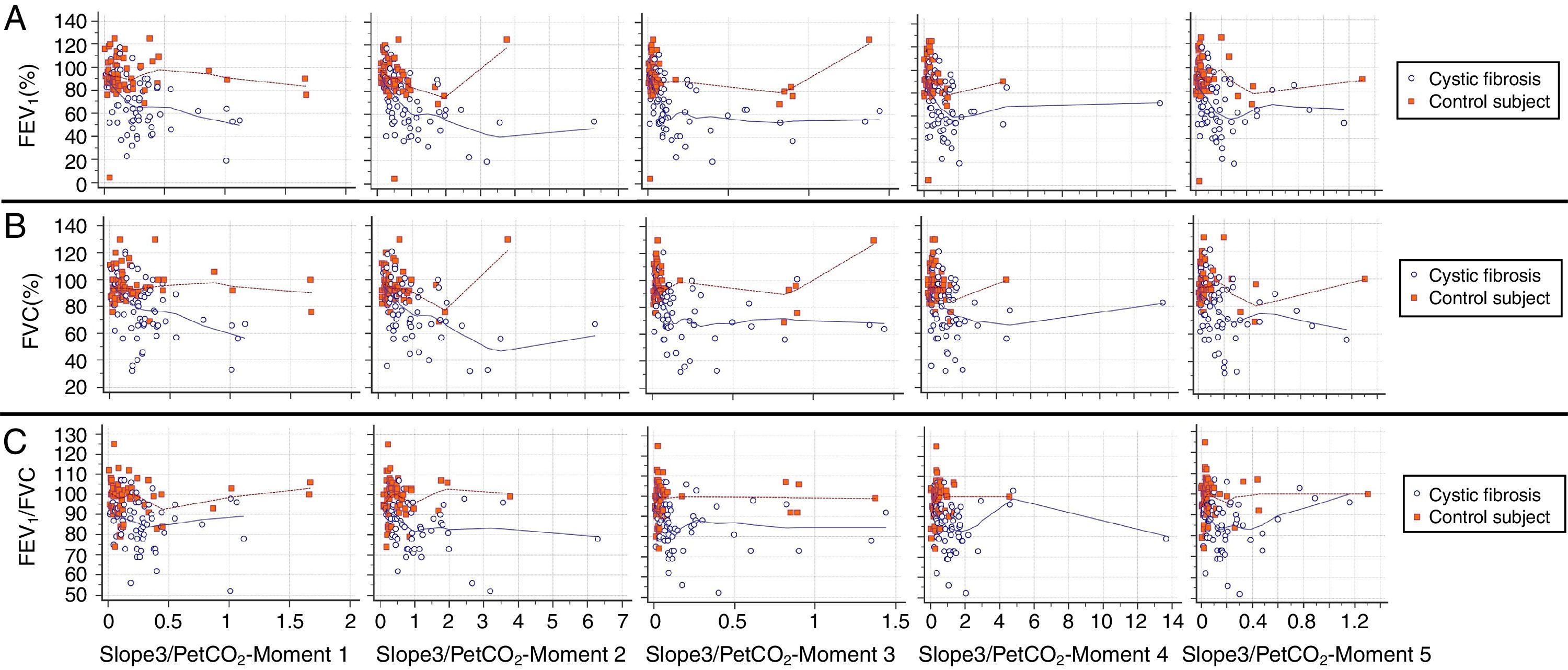
Slope 3 showed a low inverse correlation with FEV1 in both groups and at all moments of the submaximal treadmill exercise test. The FVC and FEV1/FVC ratio showed a low inverse correlation with slope 3 only for CFG (Fig. 1). Values of slope 3 corrected by the spontaneous VT and PetCO2 showed results like slope 3 analyzed separately (Figs. 2 and 3).
Correlation between slope 3 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.468**, p-value<0.001; (Moment 2) CC=−0.501**, p-value<0.001; (Moment 3) CC=−0.455**, p-value<0.001; (Moment 4) CC=−0.447**, p-value<0.001; (Moment 5) CC=−0.445**, p-value<0.001; [CFG] (Moment 1) CC=−0.338**, p-value=0.006; (Moment 2) CC=−0.471**, p-value<0.001; (Moment 3) CC=−0.462**, p-value<0.001; (Moment 4) CC=−0.388**, p-value=0.002; (Moment 5) CC=−0.293*, p-value=0.019; [CG] (Moment 1) CC=−0.277*, p-value=0.027; (Moment 2) CC=−0.305*, p-value=0.014; (Moment 3) CC=−0.124, p-value=-0.328; (Moment 4) CC=−0.267*, p-value=0.033; (Moment 5) CC=−0.245, p-value=0.051. (B) FVC: [All subjects] (Moment 1) CC=−0.376**, p-value<0.001; (Moment 2) CC=−0.394**, p-value<0.001; (Moment 3) CC=−0.365**, p-value<0.001; (Moment 4) CC=−0.359**, p-value<0.001; (Moment 5) CC=−0.330, p-value<0.001; [CFG] (Moment 1) CC=−0.315**, p-value=0.011; (Moment 2) CC=−0.411**, p-value=0.001; (Moment 3) CC=−0.407**, p-value=0.001; (Moment 4) CC=−0.354**, p-value=0.004; (Moment 5) CC=−0.236, p-value=0.061; [CG] (Moment 1) CC=−0.117, p-value=0.359; (Moment 2) CC=−0.098, p-value=0.442; (Moment 3) CC=0.098, p-value=0.439; (Moment 4) CC=−0.022, p-value=0.861; (Moment 5) CC=−0.026, p-value=0.838. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.237**, p-value=0.007; (Moment 2) CC=−0.282, p-value=0.001; (Moment 3) CC=−0.294, p-value=0.001; (Moment 4) CC=−0.284, p-value=0.001; (Moment 5) CC=−0.249, p-value=0.005; [CFG] (Moment 1) CC=−0.138, p-value=0.276; (Moment 2) CC=−0.349**, p-value=0.005; (Moment 3) CC=−0.330**, p-value=0.008; (Moment 4) CC=−0.239, p-value=0.057; (Moment 5) CC=−0.220, p-value=0.081; [CG] (Moment 1) CC=0.064, p-value=0.616; (Moment 2) CC=0.039, p-value=0.758; (Moment 3) CC=0.152, p-value=0.231; (Moment 4) CC=0.063, p-value=0.624; (Moment 5) CC=0.255*, p-value=0.042. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3, slope of phase 3 of capnography; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman's rank. Data marked with * and ** showed statistically significant p-value.
Correlation between slope 3/VT and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.437**, p-value<0.001; (Moment 2) CC=−0.454**, p-value<0.001; (Moment 3) CC=−0.422**, p-value<0.001; (Moment 4) CC=−0.431**, p-value<0.001; (Moment 5) CC=−0.409**, p-value<0.001; [CFG] (Moment 1) CC=−0.273, p-value=0.029; (Moment 2) CC=−0.428**, p-value<0.001; (Moment 3) CC=−0.406**, p-value=0.001; (Moment 4) CC=−0.351**, p-value=0.004; (Moment 5) CC=−0.213, p-value=0.091; [CG] (Moment 1) CC=−0.300*, p-value=0.016; (Moment 2) CC=−0.292*, p-value=0.019; (Moment 3) CC=−0.164, p-value=0.194; (Moment 4) CC=−0.286*, p-value=0.022; (Moment 5) CC=−0.254*, p-value=0.042. (B) FVC: [All subjects] (Moment 1) CC=−0.327**, p-value<0.001; (Moment 2) CC=−0.349**, p-value<0.001; (Moment 3) CC=−0.328**, p-value<0.001; (Moment 4) CC=−0.340**, p-value<0.001; (Moment 5) CC=−0.291**, p-value=0.001; [CFG] (Moment 1) CC=−0.256*, p-value=0.041; (Moment 2) CC=−0.378**, p-value=0.002; (Moment 3) CC=−0.362**, p-value=0.003; (Moment 4) CC=−0.327**, p-value=0.008; (Moment 5) CC=−0.146, p-value=0.250; [CG] (Moment 1) CC=−0.053, p-value=0.675; (Moment 2) CC=−0.068, p-value=0.592; (Moment 3) CC=0.083, p-value=0.512; (Moment 4) CC=−0.030, p-value=0.811; (Moment 5) CC=−0.032, p-value=0.803. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.201*, p-value=0.023; (Moment 2) CC=0.206*, p-value=0.020; (Moment 3) CC=−0.232**, p-value=0.008; (Moment 4) CC=−0.213*, p-value=0.016; (Moment 5) CC=−0.209*, p-value=0.018; [CFG] (Moment 1) CC=−0.064, p-value=0.616; (Moment 2) CC=−0.263*, p-value=0.036; (Moment 3) CC=−0.246, p-value=0.051; (Moment 4) CC=−0.168, p-value=0.185; (Moment 5) CC=−0.150, p-value=0.236; [CG] (Moment 1) CC=0.049, p-value=0.699; (Moment 2) CC=0.110, p-value=0.385; (Moment 3) CC=0.199, p-value=0.115; (Moment 4) CC=0.131, p-value=0.304; (Moment 5) CC=0.259*, p-value=0.039. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/VT, slope of phase 3 normalized by expired tidal volume; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman's rank. Data marked with * and ** showed statistically significant p-value.
Correlation between slope 3/PetCO2 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.384**, p-value<0.001; (Moment 2) CC=−0.525**, p-value<0.001; (Moment 3) CC=−0.512**, p-value<0.001; (Moment 4) CC=−0.463**, p-value<0.001; (Moment 5) CC=−0.431**, p-value<0.001; [CFG] (Moment 1) CC=−0.351**, p-value=0.005; (Moment 2) CC=−0.499**, p-value<0.001; (Moment 3) CC=−0.503, p-value<0.001; (Moment 4) CC=−0.280, p-value=0.025; (Moment 5) CC=−0.353**, p-value=0.004; [CG] (Moment 1) CC=−0.093, p-value=0.467; (Moment 2) CC=−0.313*, p-value=0.012; (Moment 3) CC=−0.173, p-value=0.172; (Moment 4) CC=−0.239, p-value=0.058; (Moment 5) CC=−0.156, p-value=0.219. B. FVC: [All subjects] (Moment 1) CC=−0.336**, p-value<0.001; (Moment 2) CC=−0.435**, p-value<0.001; (Moment 3) CC=−0.409**, p-value<0.001; (Moment 4) CC=−0.361**, p-value<0.001; (Moment 5) CC=−0.342**, p-value<0.001; [CFG] (Moment 1) CC=0.341**, p-value=0.006; (Moment 2) CC=−0.457**, p-value<0.001; (Moment 3) CC=−0.438, p-value<0.001; (Moment 4) CC=−0.238, p-value=0.058; (Moment 5) CC=−0.327**, p-value=0.008; [CG] (Moment 1) CC=0.036, p-value=0.780; (Moment 2) CC=−0.131, p-value=0.302; (Moment 3) CC=0.066, p-value=0.603; (Moment 4) CC=−0.044, p-value=0.730; (Moment 5) CC=0.044, p-value=0.727. C. FEV1/FVC: [All subjects] (Moment 1) CC=−0.292**, p-value=0.001; (Moment 2) CC=−0.298**, p-value=0.001; (Moment 3) CC=−0.289**, p-value=0.001; (Moment 4) CC=−0.275**, p-value=0.002; (Moment 5) CC=−0.252**, p-value=0.004; [CFG] (Moment 1) CC=−0.177, p-value=0.162; (Moment 2) CC=−0.346**, p-value=0.005; (Moment 3) CC=−0.228, p-value=0.070; (Moment 4) CC=−0.230, p-value=0.068; (Moment 5) CC=−0.134, p-value=0.290; [CG] (Moment 1) CC=−0.119, p-value=0.349; (Moment 2) CC=0.077, p-value=0.543; (Moment 3) CC=0.092, p-value=0.471; (Moment 4) CC=0.278*, p-value=0.026; (Moment 5) CC=0.133, p-value=0.293. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/PetCO2, slope of phase 3 normalized by end-tidal carbon dioxide tension; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman's rank. Data marked with * and ** showed statistically significant p-value.
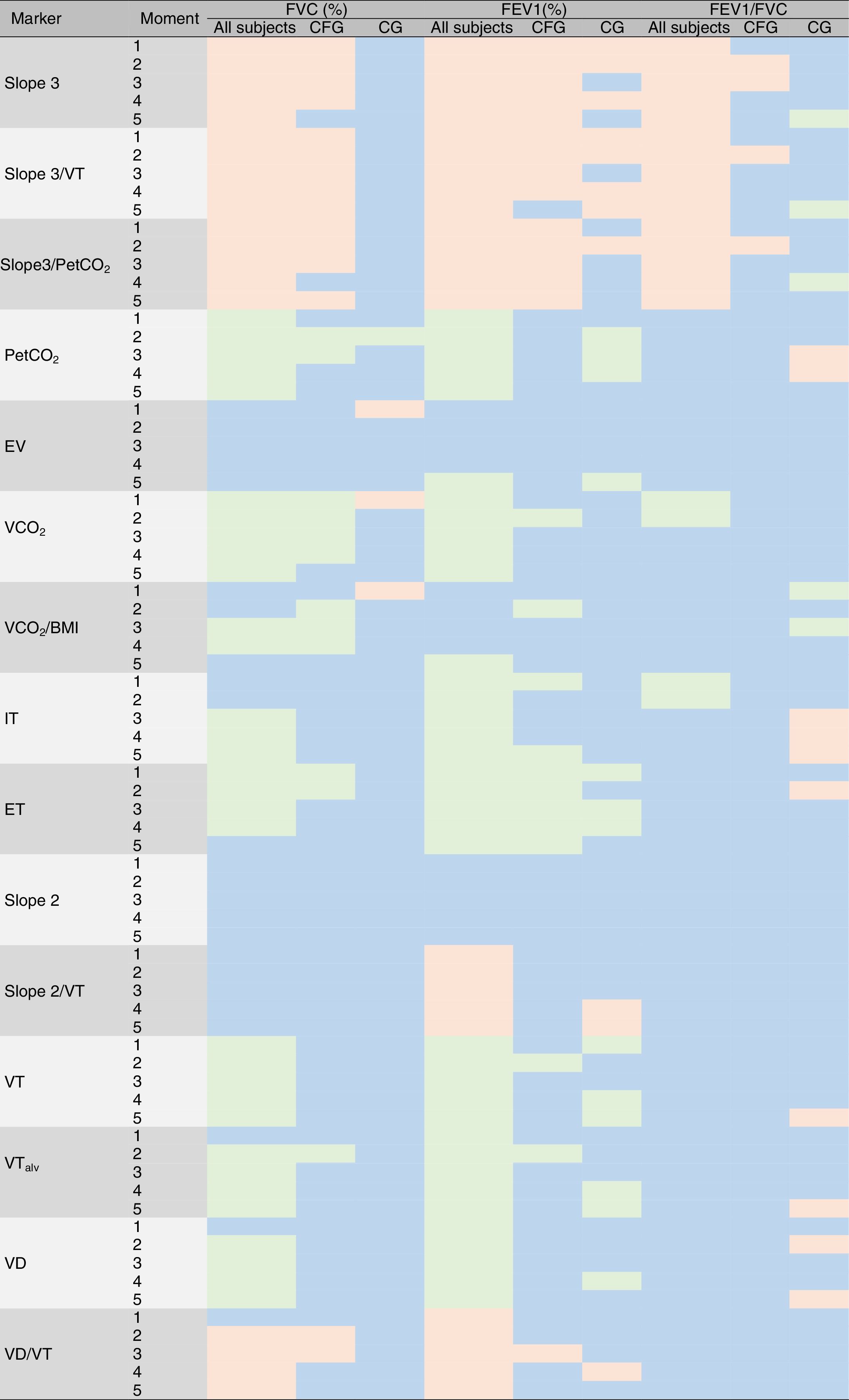
The complete data for correlations considering the VCap and spirometric markers is shown in Supplementary Material 2. Moreover, the Fig. 4 shows the descriptive analysis of all correlations performed.
Descriptive overview of correlations between variables obtained from spirometry and volumetric capnography. * G, groups; M, moments; 1, baseline time of the submaximal treadmill exercise test; 2, first and second minutes of the submaximal treadmill exercise test; 3, third and fourth minutes of the submaximal treadmill exercise test; 4, last two minutes of the submaximal treadmill exercise test; 5, measurement after the submaximal treadmill exercise test; CFG, cystic fibrosis group; CG, control group; All subjects, patients with cystic fibrosis+control subjects; IT, inspiratory time; ET, expiratory time; VT, expired tidal volume; VTalv, alveolar tidal volume; VD, anatomic dead space volume; slope 3, slope of phase 3 of capnography; slope 3/VT, slope of phase 3 normalized by VT; slope 3/PetCO2, slope of phase 3 normalized by partial pressure of carbon dioxide in the expired air; slope 2, slope of phase 2 of capnography; slope 2/VT, slope of phase 2 normalized by VT; VCO2, fraction of expired CO2; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PetCO2, partial pressure of carbon dioxide in the expired air; EV, expiratory volume. Statistical analysis was performed using Spearman correlation (Rho). Blue, no correlation was observed; red, negative correlation was observed; green, positive correlation was observed.
The search for markers with higher sensitivity and specificity to assess the damage and consequences deriving from progression of lung disease in patients with CF has been increasingly encouraged and enhanced. In this context, this seems to be the first study that correlates the prognostic indices of spirometry with promising rates of VCap.
It was noted that all indices analyzing VCap such as slope 3, slope 3/VT and slope 3/PetCO2 correlated with validated spirometric variables such as FEV1, FVC and FEV1/FVC demonstrating that VCap can identify early minor changes in airways, both at rest and mainly during exercise.
The CF causes progressive deterioration in the ability to perform physical exercise. With the progression of lung disease, the increased VD requires changes in ventilation to maintain adequate alveolar ventilation during exercise, so changes in lung function over time are correlated with changes in exercise capacity.3,4,16
On the other hand, regardless of severity, both children and adults increase the ability to tolerate and benefit from exercise in the long term.28,30 Exercise has been used as a tool to assess improvement or worsening of markers of cardiac function.29–31
In this sense, VCap, although widely used in clinical practice, is underutilized in relation to HR, probably from ignorance of the results of this clinical tool and its possibilities for practical applications.30,31
Patients with CF are constantly subjected to numerous procedures, some invasive and others which are noninvasive but require patient effort, such as spirometry. VCap comes as a counterproposal requiring minimal patient effort.4,5
Slope 3 is caused by sequential emptying of lung units with different CO2 concentrations reflecting the heterogeneity of less ventilated areas such as the lung periphery, while FEV1 reflects the most proximal airway disease.5
Ribeiro et al. (2012) by comparing the values of spirometry and VCap noted that patients with CF had higher slope 3/VT ratios, regardless of the lung disease stage, even in those with normal spirometry results, showing that VCap can identify heterogeneity of ventilation distribution in the peripheral airways of patients with CF even with normal spirometry.5 In this context, patients with CF require ventilatory increase during exercise caused by poor ventilation of the lungs resulting in heterogeneity between ventilation and perfusion, since certain regions are hypoventilated while others are hyperventilated.32–34,7
Finally, the spirometry and VCap represents different aspects/elements related to lung function [(spirometry) mainly airway obstruction; (VCap) alveolar ventilation heterogeneity and ventilation/perfusion)] and this fact can be visualized by the low correlation coefficients observed in our sample between the two tools. The lung is a complex organ and numerous tools should be used, at the same time, to perform a wide diagnostic of the lung disease – one tool is a complement to another tool.
As a study limitation, VCap reference values were not available in the literature, so we developed a curve of healthy subject values for VCap, as well as, the association regarding age and sex (e.g., these data can be done to spirometry, but the same is not true to VCap). Also, we are not able to perform multiple linear regression procedure regarding the low number of patients with CF and healthy control subjects enrolled and the correlation analysis providing a number that summarizes the degree of linear relationship between the two variables (spirometry and VCap). Also, the regression analysis provides an equation that describes the behavior of one of the variables as a function of the behavior of the other variable, which is not known in VCap. Thus, to date, knowledge about the cause and effect factor between both tools is limited and the markers of each tool appear to reflect on different phenotypic anomalies of the disease. Thus, in this first step, we believe that the interpretation, even with caution, of the correlation, and later on the regression, is more relevant. In addition, the interpretation of the results with low coefficient correlations and the limits of linear regression method that informs on the intensity of the liaison between two variables is limited, and new studies should be done to prove our findings. But, VCap tracking and monitoring of lung function in patients with CF may be a promising parameter. Also, follow-up studies may show more evidence of the effectiveness of the management of VCap in CF and other diseases. In addition, the VCap shows some limitations in discriminating the severity of the small airways alteration and it may be worth exploring another index to describe these alterations. In this way, studies should be performed including several indexes achieved from VCap, as well, the comparison between it and some lung function methods.
Although VCap is a tool used mainly for monitoring critically ill and mechanically ventilated subjects, it has been proven to be a valuable tool in the evaluation of patients with CF before and during the exercise. The VCap differences and advantages include the lack of need for forced breathing maneuvers and, unlike spirometry, the possible elimination of bias such as patient effort, technical expertise and reference equations.
ConclusionThe VCap indices had low correlation with parameter already validated in spirometry during submaximal treadmill exercise test such as FEV1, FVC and FEV1/FVC demonstrating that VCap is a useful tool for evaluating the pulmonary status of children and adolescents with CF being a complementary tool – spirometry (airway obstruction) and VCap (alveolar ventilation heterogeneity and ventilation/perfusion) methods do not assess the same element. The use of VCap and application associated with physical exercise provides information about respiratory behavior during this activity in these subjects, and the presence of obstruction, even in patients with unchanged FEV1.
Ethical approvalThe study followed the ethical standards of the Helsinki agreement and was approved by the Ethics Committee of the institution (#1182/2009).
Funding informationPaloma L.F. Parazzi: Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq); Fernando A.L. Marson: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the studies #2011/12939-4 and #2015/12858-5; Fundo de Apoio à Pesquisa ao Ensino e à Extensão da Universidade Estadual de Campinas for the study #0648/2015; José D. Ribeiro: FAPESP for the study #2011/18845-1 and #2015/12183-8.
Authors’ contributionPLFP, FALM, MAGOR, CISS, JDR – made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; involved in drafting the manuscript and revising it critically for important intellectual content; gave final approval of the manuscript version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Conflicts of interestThe authors declare that there is no conflict of interest.
The authors thank all who participated as subjects in this study and in a way contributed to its completion.



![Correlation between slope 3 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.468**, p-value<0.001; (Moment 2) CC=−0.501**, p-value<0.001; (Moment 3) CC=−0.455**, p-value<0.001; (Moment 4) CC=−0.447**, p-value<0.001; (Moment 5) CC=−0.445**, p-value<0.001; [CFG] (Moment 1) CC=−0.338**, p-value=0.006; (Moment 2) CC=−0.471**, p-value<0.001; (Moment 3) CC=−0.462**, p-value<0.001; (Moment 4) CC=−0.388**, p-value=0.002; (Moment 5) CC=−0.293*, p-value=0.019; [CG] (Moment 1) CC=−0.277*, p-value=0.027; (Moment 2) CC=−0.305*, p-value=0.014; (Moment 3) CC=−0.124, p-value=-0.328; (Moment 4) CC=−0.267*, p-value=0.033; (Moment 5) CC=−0.245, p-value=0.051. (B) FVC: [All subjects] (Moment 1) CC=−0.376**, p-value<0.001; (Moment 2) CC=−0.394**, p-value<0.001; (Moment 3) CC=−0.365**, p-value<0.001; (Moment 4) CC=−0.359**, p-value<0.001; (Moment 5) CC=−0.330, p-value<0.001; [CFG] (Moment 1) CC=−0.315**, p-value=0.011; (Moment 2) CC=−0.411**, p-value=0.001; (Moment 3) CC=−0.407**, p-value=0.001; (Moment 4) CC=−0.354**, p-value=0.004; (Moment 5) CC=−0.236, p-value=0.061; [CG] (Moment 1) CC=−0.117, p-value=0.359; (Moment 2) CC=−0.098, p-value=0.442; (Moment 3) CC=0.098, p-value=0.439; (Moment 4) CC=−0.022, p-value=0.861; (Moment 5) CC=−0.026, p-value=0.838. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.237**, p-value=0.007; (Moment 2) CC=−0.282, p-value=0.001; (Moment 3) CC=−0.294, p-value=0.001; (Moment 4) CC=−0.284, p-value=0.001; (Moment 5) CC=−0.249, p-value=0.005; [CFG] (Moment 1) CC=−0.138, p-value=0.276; (Moment 2) CC=−0.349**, p-value=0.005; (Moment 3) CC=−0.330**, p-value=0.008; (Moment 4) CC=−0.239, p-value=0.057; (Moment 5) CC=−0.220, p-value=0.081; [CG] (Moment 1) CC=0.064, p-value=0.616; (Moment 2) CC=0.039, p-value=0.758; (Moment 3) CC=0.152, p-value=0.231; (Moment 4) CC=0.063, p-value=0.624; (Moment 5) CC=0.255*, p-value=0.042. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3, slope of phase 3 of capnography; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman Correlation between slope 3 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.468**, p-value<0.001; (Moment 2) CC=−0.501**, p-value<0.001; (Moment 3) CC=−0.455**, p-value<0.001; (Moment 4) CC=−0.447**, p-value<0.001; (Moment 5) CC=−0.445**, p-value<0.001; [CFG] (Moment 1) CC=−0.338**, p-value=0.006; (Moment 2) CC=−0.471**, p-value<0.001; (Moment 3) CC=−0.462**, p-value<0.001; (Moment 4) CC=−0.388**, p-value=0.002; (Moment 5) CC=−0.293*, p-value=0.019; [CG] (Moment 1) CC=−0.277*, p-value=0.027; (Moment 2) CC=−0.305*, p-value=0.014; (Moment 3) CC=−0.124, p-value=-0.328; (Moment 4) CC=−0.267*, p-value=0.033; (Moment 5) CC=−0.245, p-value=0.051. (B) FVC: [All subjects] (Moment 1) CC=−0.376**, p-value<0.001; (Moment 2) CC=−0.394**, p-value<0.001; (Moment 3) CC=−0.365**, p-value<0.001; (Moment 4) CC=−0.359**, p-value<0.001; (Moment 5) CC=−0.330, p-value<0.001; [CFG] (Moment 1) CC=−0.315**, p-value=0.011; (Moment 2) CC=−0.411**, p-value=0.001; (Moment 3) CC=−0.407**, p-value=0.001; (Moment 4) CC=−0.354**, p-value=0.004; (Moment 5) CC=−0.236, p-value=0.061; [CG] (Moment 1) CC=−0.117, p-value=0.359; (Moment 2) CC=−0.098, p-value=0.442; (Moment 3) CC=0.098, p-value=0.439; (Moment 4) CC=−0.022, p-value=0.861; (Moment 5) CC=−0.026, p-value=0.838. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.237**, p-value=0.007; (Moment 2) CC=−0.282, p-value=0.001; (Moment 3) CC=−0.294, p-value=0.001; (Moment 4) CC=−0.284, p-value=0.001; (Moment 5) CC=−0.249, p-value=0.005; [CFG] (Moment 1) CC=−0.138, p-value=0.276; (Moment 2) CC=−0.349**, p-value=0.005; (Moment 3) CC=−0.330**, p-value=0.008; (Moment 4) CC=−0.239, p-value=0.057; (Moment 5) CC=−0.220, p-value=0.081; [CG] (Moment 1) CC=0.064, p-value=0.616; (Moment 2) CC=0.039, p-value=0.758; (Moment 3) CC=0.152, p-value=0.231; (Moment 4) CC=0.063, p-value=0.624; (Moment 5) CC=0.255*, p-value=0.042. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3, slope of phase 3 of capnography; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman](https://static.elsevier.es/multimedia/25310437/0000002500000001/v1_201902260629/S2531043718300758/v1_201902260629/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)
![Correlation between slope 3/VT and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.437**, p-value<0.001; (Moment 2) CC=−0.454**, p-value<0.001; (Moment 3) CC=−0.422**, p-value<0.001; (Moment 4) CC=−0.431**, p-value<0.001; (Moment 5) CC=−0.409**, p-value<0.001; [CFG] (Moment 1) CC=−0.273, p-value=0.029; (Moment 2) CC=−0.428**, p-value<0.001; (Moment 3) CC=−0.406**, p-value=0.001; (Moment 4) CC=−0.351**, p-value=0.004; (Moment 5) CC=−0.213, p-value=0.091; [CG] (Moment 1) CC=−0.300*, p-value=0.016; (Moment 2) CC=−0.292*, p-value=0.019; (Moment 3) CC=−0.164, p-value=0.194; (Moment 4) CC=−0.286*, p-value=0.022; (Moment 5) CC=−0.254*, p-value=0.042. (B) FVC: [All subjects] (Moment 1) CC=−0.327**, p-value<0.001; (Moment 2) CC=−0.349**, p-value<0.001; (Moment 3) CC=−0.328**, p-value<0.001; (Moment 4) CC=−0.340**, p-value<0.001; (Moment 5) CC=−0.291**, p-value=0.001; [CFG] (Moment 1) CC=−0.256*, p-value=0.041; (Moment 2) CC=−0.378**, p-value=0.002; (Moment 3) CC=−0.362**, p-value=0.003; (Moment 4) CC=−0.327**, p-value=0.008; (Moment 5) CC=−0.146, p-value=0.250; [CG] (Moment 1) CC=−0.053, p-value=0.675; (Moment 2) CC=−0.068, p-value=0.592; (Moment 3) CC=0.083, p-value=0.512; (Moment 4) CC=−0.030, p-value=0.811; (Moment 5) CC=−0.032, p-value=0.803. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.201*, p-value=0.023; (Moment 2) CC=0.206*, p-value=0.020; (Moment 3) CC=−0.232**, p-value=0.008; (Moment 4) CC=−0.213*, p-value=0.016; (Moment 5) CC=−0.209*, p-value=0.018; [CFG] (Moment 1) CC=−0.064, p-value=0.616; (Moment 2) CC=−0.263*, p-value=0.036; (Moment 3) CC=−0.246, p-value=0.051; (Moment 4) CC=−0.168, p-value=0.185; (Moment 5) CC=−0.150, p-value=0.236; [CG] (Moment 1) CC=0.049, p-value=0.699; (Moment 2) CC=0.110, p-value=0.385; (Moment 3) CC=0.199, p-value=0.115; (Moment 4) CC=0.131, p-value=0.304; (Moment 5) CC=0.259*, p-value=0.039. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/VT, slope of phase 3 normalized by expired tidal volume; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman Correlation between slope 3/VT and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.437**, p-value<0.001; (Moment 2) CC=−0.454**, p-value<0.001; (Moment 3) CC=−0.422**, p-value<0.001; (Moment 4) CC=−0.431**, p-value<0.001; (Moment 5) CC=−0.409**, p-value<0.001; [CFG] (Moment 1) CC=−0.273, p-value=0.029; (Moment 2) CC=−0.428**, p-value<0.001; (Moment 3) CC=−0.406**, p-value=0.001; (Moment 4) CC=−0.351**, p-value=0.004; (Moment 5) CC=−0.213, p-value=0.091; [CG] (Moment 1) CC=−0.300*, p-value=0.016; (Moment 2) CC=−0.292*, p-value=0.019; (Moment 3) CC=−0.164, p-value=0.194; (Moment 4) CC=−0.286*, p-value=0.022; (Moment 5) CC=−0.254*, p-value=0.042. (B) FVC: [All subjects] (Moment 1) CC=−0.327**, p-value<0.001; (Moment 2) CC=−0.349**, p-value<0.001; (Moment 3) CC=−0.328**, p-value<0.001; (Moment 4) CC=−0.340**, p-value<0.001; (Moment 5) CC=−0.291**, p-value=0.001; [CFG] (Moment 1) CC=−0.256*, p-value=0.041; (Moment 2) CC=−0.378**, p-value=0.002; (Moment 3) CC=−0.362**, p-value=0.003; (Moment 4) CC=−0.327**, p-value=0.008; (Moment 5) CC=−0.146, p-value=0.250; [CG] (Moment 1) CC=−0.053, p-value=0.675; (Moment 2) CC=−0.068, p-value=0.592; (Moment 3) CC=0.083, p-value=0.512; (Moment 4) CC=−0.030, p-value=0.811; (Moment 5) CC=−0.032, p-value=0.803. (C) FEV1/FVC: [All subjects] (Moment 1) CC=−0.201*, p-value=0.023; (Moment 2) CC=0.206*, p-value=0.020; (Moment 3) CC=−0.232**, p-value=0.008; (Moment 4) CC=−0.213*, p-value=0.016; (Moment 5) CC=−0.209*, p-value=0.018; [CFG] (Moment 1) CC=−0.064, p-value=0.616; (Moment 2) CC=−0.263*, p-value=0.036; (Moment 3) CC=−0.246, p-value=0.051; (Moment 4) CC=−0.168, p-value=0.185; (Moment 5) CC=−0.150, p-value=0.236; [CG] (Moment 1) CC=0.049, p-value=0.699; (Moment 2) CC=0.110, p-value=0.385; (Moment 3) CC=0.199, p-value=0.115; (Moment 4) CC=0.131, p-value=0.304; (Moment 5) CC=0.259*, p-value=0.039. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/VT, slope of phase 3 normalized by expired tidal volume; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman](https://static.elsevier.es/multimedia/25310437/0000002500000001/v1_201902260629/S2531043718300758/v1_201902260629/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)
![Correlation between slope 3/PetCO2 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.384**, p-value<0.001; (Moment 2) CC=−0.525**, p-value<0.001; (Moment 3) CC=−0.512**, p-value<0.001; (Moment 4) CC=−0.463**, p-value<0.001; (Moment 5) CC=−0.431**, p-value<0.001; [CFG] (Moment 1) CC=−0.351**, p-value=0.005; (Moment 2) CC=−0.499**, p-value<0.001; (Moment 3) CC=−0.503, p-value<0.001; (Moment 4) CC=−0.280, p-value=0.025; (Moment 5) CC=−0.353**, p-value=0.004; [CG] (Moment 1) CC=−0.093, p-value=0.467; (Moment 2) CC=−0.313*, p-value=0.012; (Moment 3) CC=−0.173, p-value=0.172; (Moment 4) CC=−0.239, p-value=0.058; (Moment 5) CC=−0.156, p-value=0.219. B. FVC: [All subjects] (Moment 1) CC=−0.336**, p-value<0.001; (Moment 2) CC=−0.435**, p-value<0.001; (Moment 3) CC=−0.409**, p-value<0.001; (Moment 4) CC=−0.361**, p-value<0.001; (Moment 5) CC=−0.342**, p-value<0.001; [CFG] (Moment 1) CC=0.341**, p-value=0.006; (Moment 2) CC=−0.457**, p-value<0.001; (Moment 3) CC=−0.438, p-value<0.001; (Moment 4) CC=−0.238, p-value=0.058; (Moment 5) CC=−0.327**, p-value=0.008; [CG] (Moment 1) CC=0.036, p-value=0.780; (Moment 2) CC=−0.131, p-value=0.302; (Moment 3) CC=0.066, p-value=0.603; (Moment 4) CC=−0.044, p-value=0.730; (Moment 5) CC=0.044, p-value=0.727. C. FEV1/FVC: [All subjects] (Moment 1) CC=−0.292**, p-value=0.001; (Moment 2) CC=−0.298**, p-value=0.001; (Moment 3) CC=−0.289**, p-value=0.001; (Moment 4) CC=−0.275**, p-value=0.002; (Moment 5) CC=−0.252**, p-value=0.004; [CFG] (Moment 1) CC=−0.177, p-value=0.162; (Moment 2) CC=−0.346**, p-value=0.005; (Moment 3) CC=−0.228, p-value=0.070; (Moment 4) CC=−0.230, p-value=0.068; (Moment 5) CC=−0.134, p-value=0.290; [CG] (Moment 1) CC=−0.119, p-value=0.349; (Moment 2) CC=0.077, p-value=0.543; (Moment 3) CC=0.092, p-value=0.471; (Moment 4) CC=0.278*, p-value=0.026; (Moment 5) CC=0.133, p-value=0.293. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/PetCO2, slope of phase 3 normalized by end-tidal carbon dioxide tension; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman Correlation between slope 3/PetCO2 and spirometry markers. (A) FEV1: [All subjects] (Moment 1) CC=−0.384**, p-value<0.001; (Moment 2) CC=−0.525**, p-value<0.001; (Moment 3) CC=−0.512**, p-value<0.001; (Moment 4) CC=−0.463**, p-value<0.001; (Moment 5) CC=−0.431**, p-value<0.001; [CFG] (Moment 1) CC=−0.351**, p-value=0.005; (Moment 2) CC=−0.499**, p-value<0.001; (Moment 3) CC=−0.503, p-value<0.001; (Moment 4) CC=−0.280, p-value=0.025; (Moment 5) CC=−0.353**, p-value=0.004; [CG] (Moment 1) CC=−0.093, p-value=0.467; (Moment 2) CC=−0.313*, p-value=0.012; (Moment 3) CC=−0.173, p-value=0.172; (Moment 4) CC=−0.239, p-value=0.058; (Moment 5) CC=−0.156, p-value=0.219. B. FVC: [All subjects] (Moment 1) CC=−0.336**, p-value<0.001; (Moment 2) CC=−0.435**, p-value<0.001; (Moment 3) CC=−0.409**, p-value<0.001; (Moment 4) CC=−0.361**, p-value<0.001; (Moment 5) CC=−0.342**, p-value<0.001; [CFG] (Moment 1) CC=0.341**, p-value=0.006; (Moment 2) CC=−0.457**, p-value<0.001; (Moment 3) CC=−0.438, p-value<0.001; (Moment 4) CC=−0.238, p-value=0.058; (Moment 5) CC=−0.327**, p-value=0.008; [CG] (Moment 1) CC=0.036, p-value=0.780; (Moment 2) CC=−0.131, p-value=0.302; (Moment 3) CC=0.066, p-value=0.603; (Moment 4) CC=−0.044, p-value=0.730; (Moment 5) CC=0.044, p-value=0.727. C. FEV1/FVC: [All subjects] (Moment 1) CC=−0.292**, p-value=0.001; (Moment 2) CC=−0.298**, p-value=0.001; (Moment 3) CC=−0.289**, p-value=0.001; (Moment 4) CC=−0.275**, p-value=0.002; (Moment 5) CC=−0.252**, p-value=0.004; [CFG] (Moment 1) CC=−0.177, p-value=0.162; (Moment 2) CC=−0.346**, p-value=0.005; (Moment 3) CC=−0.228, p-value=0.070; (Moment 4) CC=−0.230, p-value=0.068; (Moment 5) CC=−0.134, p-value=0.290; [CG] (Moment 1) CC=−0.119, p-value=0.349; (Moment 2) CC=0.077, p-value=0.543; (Moment 3) CC=0.092, p-value=0.471; (Moment 4) CC=0.278*, p-value=0.026; (Moment 5) CC=0.133, p-value=0.293. CC, correlation coefficient; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; slope 3/PetCO2, slope of phase 3 normalized by end-tidal carbon dioxide tension; CFG, group with cystic fibrosis (N=64); CG, control group (N=64). All subjects, patients with cystic fibrosis+control subjects (N=128). ** The correlation is significant at the 0.01 level (2 ends). * The correlation is significant at the 0.05 level (2 ends). Alpha=0.05. Statistical analysis was performed using Spearman correlation (Rho). In the legend is presented the correlation coefficient of Spearman](https://static.elsevier.es/multimedia/25310437/0000002500000001/v1_201902260629/S2531043718300758/v1_201902260629/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)





