Asthma affects the lives of several hundred million people around the World, across all age groups and Portugal is not an exception, with an asthma lifetime prevalence of 10.5%.1 Patients, their families and the society face high direct and indirect costs, due to healthcare resources use, loss of productivity, absenteeism and presenteeism of patients. Asthma strongly influences the wellbeing and quality of life of patients. With no established curative treatment, guidelines for asthma management have identified that the primary goal of management is to achieve asthma control.2 In asthma, pharmacological treatments mainly consist of inhaled drugs, which allow efficacy and significantly reduce systemic side effects, being the most efficacious treatments. However, lack of adherence to treatment in asthma occurs in more than half of all medical prescriptions. Furthermore, reduced adherence to treatment frequently allies with an incorrect inhalation technique, both considered major issues significantly impairing pharmacologic treatments effectiveness. Therefore, despite the efficacy of the available drugs, a high percentage of asthmatics are uncontrolled and have frequent exacerbations.
Difficulties in using inhalation methods are well-known problems that have been consistently maintained in recent decades, with the occurrence of several errors that affect treatment results with the use of both dry powder inhalers (DPIs) and pressurized metered-dose inhalers (pMDIs). Results from the Critikal study3 analyses of the inhaler technique assessment initiative Helping Asthma in Real-life Patients (iHARP) database have helped to identify the prevalence of critical inhaler errors (those that have a definite detrimental impact on the delivery of drug to the lungs) with different devices in patients with asthma. The most common critical errors included failure to coordinate device actuation and inhalation with pMDIs, and lack of a forceful inhalation with DPIs; overall, 89% of patients made at least one potentially critical handling error and 67% made multiple potentially critical errors.3
Breath-actuated inhalers (BAIs) represented an evolution in inhalers’ design aiming to improve the management of asthma.4 BAIs are aerosol devices, like pMDIs, but rather than being activated manually, they automatically release a dose of drug when the patient inhales with a sufficient inhalation flow rate. In the past, BAIs had a major limitation when chlorofluorocarbon propellants (CFCs) were used and the speed of emission of the aerosol was so high that the impact of inhaled droplets in the upper airways was still very significant; nowadays, with hydrofluoroalkane propellants (HFAs), the speed of emission is considerably lower and the aerosol may be inhaled slowly being deposited more peripherally in the airways. These new devices may offer several advantages (Table 1), and were developed to overcome the most commonly seen critical errors with other inhalers, as follows: there is no need to coordinate actuation and inhalation (which is necessary for pMDIs); as active devices, BAIs emit a propelled aerosol and patients do not need to inhale forcibly to generate respirable particles (which is required for DPIs).4,5
Breath-actuated inhalers: key advantages and disadvantages.
| Advantages | Disadvantages |
|---|---|
| Portable and compact• Multi-dose device• High reproducibility in the amount of drug delivered• Closed canister, so contents cannot be contaminatedBenefits over conventional pMDIs– No need to coordinate inhalation and actuationBenefits over DPIs– Releases drug at low inspiratory force, so no need to inhale forcibly | • Available for a limited range of drugs• Suspension formulations need to be shaken before each use• Important to prime before first use, if not used for some time or if the inhaler has been exposed to cold temperatures |
BAIs are intended to simplify the inhaler technique, leading to improved inhaler use by the patients and less health care professional (HCP) time spent training patients to use the devices correctly.4 Indeed, several studies have shown that patients find BAIs easier to use and HCP find it easier to train patients in their correct use in relation to other devices. The ease of use of BAIs may offer particular advantages in certain patient groups, such as children, the elderly or those with limited manual dexterity.4,5 BAIs that are triggered by a low inspiratory force may offer additional advantage.4
An ergonomically designed breath-triggered inhaler (BTI), k-haler®,5 was recently available. Its successful use involves only a few steps and, as an ‘active’ aerosol inhaler, it automatically releases a dose of the drug in a respirable form when a patient inhales, even at a low inspiratory flow (the device is triggered at an inspiratory flow rate of approximately 30L/min). A high fine particle fraction and a plume that is less forceful than that of previous pMDIs can decrease drug impaction at the back of the throat and improve delivery to the lungs.5 As such, k-haler® represents an added-value to improve asthma control by addressing current patients’ needs and overcoming the most common lasting critical errors referred with other inhalers (Table 2).5
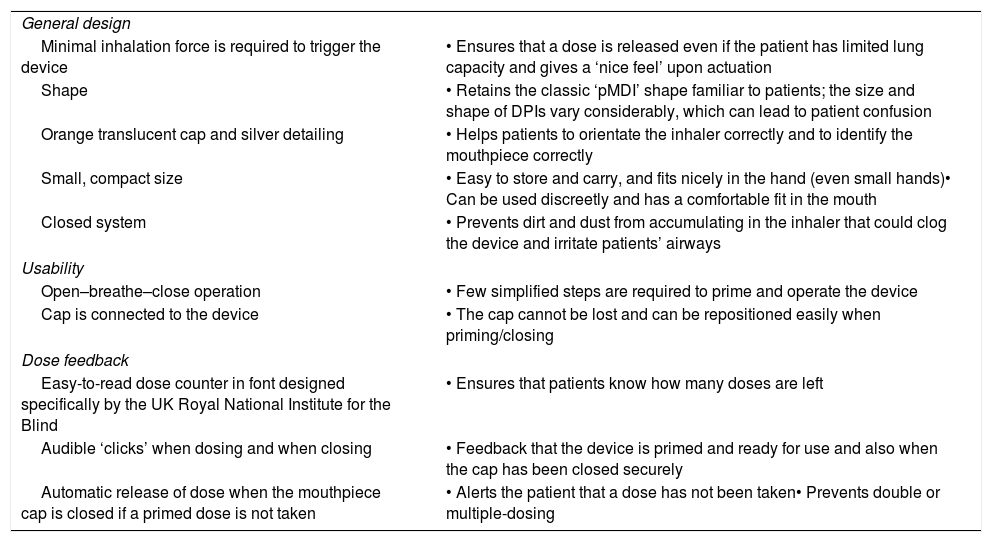
Key attributes of the breath-triggered k-haler®.
| General design | |
| Minimal inhalation force is required to trigger the device | • Ensures that a dose is released even if the patient has limited lung capacity and gives a ‘nice feel’ upon actuation |
| Shape | • Retains the classic ‘pMDI’ shape familiar to patients; the size and shape of DPIs vary considerably, which can lead to patient confusion |
| Orange translucent cap and silver detailing | • Helps patients to orientate the inhaler correctly and to identify the mouthpiece correctly |
| Small, compact size | • Easy to store and carry, and fits nicely in the hand (even small hands)• Can be used discreetly and has a comfortable fit in the mouth |
| Closed system | • Prevents dirt and dust from accumulating in the inhaler that could clog the device and irritate patients’ airways |
| Usability | |
| Open–breathe–close operation | • Few simplified steps are required to prime and operate the device |
| Cap is connected to the device | • The cap cannot be lost and can be repositioned easily when priming/closing |
| Dose feedback | |
| Easy-to-read dose counter in font designed specifically by the UK Royal National Institute for the Blind | • Ensures that patients know how many doses are left |
| Audible ‘clicks’ when dosing and when closing | • Feedback that the device is primed and ready for use and also when the cap has been closed securely |
| Automatic release of dose when the mouthpiece cap is closed if a primed dose is not taken | • Alerts the patient that a dose has not been taken• Prevents double or multiple-dosing |
The simplicity of use, better inhaler handling and patient preference for BAIs are advantages that may translate into improved treatment compliance with the prescribed therapy, leading to improved lung function and asthma control compared with other devices.4–6 Several controlled studies comparing the efficacy of drugs at equivalent nominal doses administered with different devices had demonstrated equivalence in the main clinical outcomes (mostly symptoms and exacerbations) of asthma (or chronic obstructive pulmonary disease), but this may be justified by, 1. the large number of variables affecting the clinical response to inhaled drugs, besides the inhalation technique and 2. the gap between the patients in clinical trial conditions in referral centers with specific characteristics and close monitoring, being aware that they are being evaluated, and those patients in the real world, where poor inhalation technique and low adherence to therapy are known to be more common.3–7
If correctly and effectively used, inhalers are excellent, safe and effective in controlling asthma, as in other chronic respiratory diseases.7 Improving inhalers correct and effective use is therefore a global issue to overcome current known difficulties and to move forward into achieving higher rates of asthma control.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this article.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingNone to declare.
Conflicts of interestNone to declare.