To investigate the frequency of lung cancer in patients with pulmonary hamartomas and to evaluate clinical, radiological, and pathological characteristics of pulmonary hamartomas.
Basic proceduresWe reviewed pathology records of pulmonary hamartomas diagnosed between 2003 and 2014. Medical records and the hospital electronic database were also reviewed for each patient to obtain clinical, radiological, and pathological characteristics of pulmonary hamartomas and accompanying malignancies.
Main findingsNinety-six patients with pulmonary hamartomas were identified. There were 26 females (27%) and 70 males (73%), with a mean age of 56.2 years (range 22–87 years). Malignancies were detected in 23 patients (24%), which developed previously in five patients (1 synchronous, 4 metachronous lesions), and concomitantly in 18 patients (with origin from the lung in 17 patients and from the pleura in 1 patient).
Principal conclusionsOur results show that patients with pulmonary hamartomas may have coexisting lung malignancies.
The term hamartoma was first introduced by Albrecht in 1904 to describe lesions that contain an abnormal mixture of tissue elements or an abnormal proportion of a single element, normally present in an organ. While hamartoma was initially considered a development malformation, it is now classified as a true benign mesenchymal tumor, consisting of cartilage, fat, fibromyxoid connective tissue, smooth muscle, and bone.1,2 Hamartomas can occur in any organ or region. Pulmonary hamartoma is the most common benign tumor of the lung, accounting for 3% of all lung tumors.3 Its incidence was found as 0.025% and 0.32% in two large autopsy-based studies.4
There have been many reports suggesting an association between hamartoma and malignancy,5–10 and the likelihood of its malignant transformation to carcinoma or sarcoma.5–7 The risk for lung cancer in pulmonary hamartoma patients was estimated to be 6.3 times as high as that expected for the general population after adjustment for age, sex, and ethnicity.8 Additionally, patients with pulmonary hamartomas can develop synchronous or metachronous lung cancers.9,10 The aim of this study was to investigate the frequency of lung cancer in patients with pulmonary hamartomas and to evaluate clinical, radiological, and pathological characteristics of pulmonary hamartomas accompanied by lung cancer.
Materials and methodsThis retrospective study was conducted at Süreyyapaşa Chest Diseases and Thoracic Surgery Training and Research Hospital, İstanbul, Turkey, after being approved by the scientific committee of our institute. A comprehensive review of pathology records was made for pulmonary hamartomas diagnosed between 2003 and 2014, which yielded a total of 97 patients. All histologic slides were re-examined by a pathologist experienced in thoracic pathology to re-confirm the diagnosis of pulmonary hamartomas. One patient whose diagnosis could not be confirmed was excluded from the study. Thus, the study included 96 patients with pulmonary hamartomas.
Medical records and the hospital electronic database were reviewed for each patient and a standardized data-collection form was used to collect the following information: gender, age at diagnosis, history of smoking, medical history, the presence of malignancies (previous, concurrent), symptoms, radiological and imaging findings, location and size of hamartomas, findings of preoperative diagnostic investigations, duration between diagnosis of malignancy and pulmonary hamartoma, malignancy staging, treatment modalities, histologic features, duration of follow-up, and outcome. Follow-up information included data till January 2015.
ResultsOf 96 patients with pulmonary hamartomas, 26 (27%) were females and 70 (73%) were males, with a female-to-male ratio of 1:2.7. The mean age was 56.2 years (range 22–87 years). Only one patient was younger than 30 years. Sixty-one patients (63.5%) were between 30 and 60 years and 34 patients (35.4%) were older than 60 years. With respect to smoking, 62 patients (64.6%) were smokers (mean 41.2 pack-years), and 34 (35.4%) had never smoked.
At presentation, 27 patients (28%) were symptom-free, while the remaining 69 patients (72%) had pulmonary and/or constitutional symptoms. The presenting symptoms were cough (n=27), chest pain (n=26), dyspnea (n=23), hemoptysis (n=10), fever (n=7), sputum production (n=5), and constitutional symptoms (n=7). The duration of symptoms ranged from 4 days to 1 year.
Chest X-ray examination was performed in all patients, which showed abnormal findings in all but three patients. Eighteen patients also had findings associated with concomitant malignancies. All patients underwent computed tomography and bronchoscopic examinations. The diagnoses of pulmonary hamartomas were established via bronchoscopic biopsies in 19 patients, computed tomography-guided transthoracic fine-needle aspiration in three patients, and surgical biopsies in 74 patients. A single hamartoma was found in 93 patients (97%) which was located in the right lung in 54 patients (58%) and in the left lung in 39 patients (42%). Three patients (3%) had multiple hamartomas, with two patients having two hamartomas in the right and left lungs, and one patient having three hamartomas in the same lobe. The hamartomas were parenchymal in 72 patients (75%) and endobronchial in 24 patients (25%). The mean diameter of pulmonary hamartomas was 22.2mm (range 3mm–100mm).
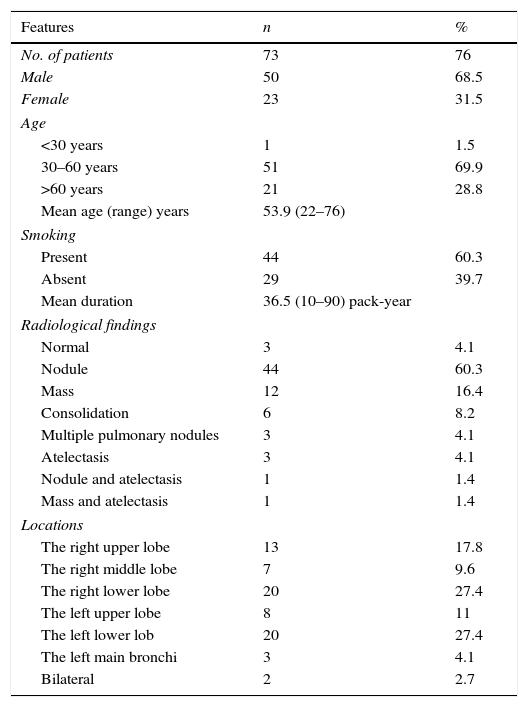
There was no evidence for malignancy in 73 patients (76%) including 50 males and 23 females, with a mean age of 53.9 years (range 22–76 years). Data on these patients are summarized in Table 1. Of these, 44 patients (60.3%) were smokers and 50 patients (68.5%) were symptomatic at presentation. The most common radiological pattern was a solitary pulmonary nodule, followed by a solitary mass. Seventy patients (95.9%) had a single hamartoma and three patients (4.1%) had multiple hamartomas. The hamartomas were parenchymal in 52 patients (71.2%) and endobronchial in 21 patients (28.8%). The diameter of pulmonary hamartomas ranged from 6mm to 100mm (mean 25.4mm). 18Fluoro-deoxyglucose positron emission tomography-computed tomography (FDG PET-CT) was performed in 28 patients. Maximum standard uptake values (SUVmax) ranged from ametabolic to 9.7. Of 73 patients without malignancies, four patients rejected treatment. Endobronchial treatment was performed in nine patients, including rigid forceps (n=3), cryotherapy (n=3), electrocautery (n=2), and argon plasma coagulation (n=1). Sixty patients underwent surgical treatment which included wedge resection (n=26), extirpation (n=14), lobectomy (n=11), enucleation (n=4), bronchotomy (n=3), and segmentectomy (n=2). Sixty-five patients (89%) were followed for a mean duration of 67.1 months (range 4–131 months). None of these patients had evidence of recurrence or developed subsequent malignancies.
Characteristics of the hamartoma patients without any malignancy.
| Features | n | % |
|---|---|---|
| No. of patients | 73 | 76 |
| Male | 50 | 68.5 |
| Female | 23 | 31.5 |
| Age | ||
| <30 years | 1 | 1.5 |
| 30–60 years | 51 | 69.9 |
| >60 years | 21 | 28.8 |
| Mean age (range) years | 53.9 (22–76) | |
| Smoking | ||
| Present | 44 | 60.3 |
| Absent | 29 | 39.7 |
| Mean duration | 36.5 (10–90) pack-year | |
| Radiological findings | ||
| Normal | 3 | 4.1 |
| Nodule | 44 | 60.3 |
| Mass | 12 | 16.4 |
| Consolidation | 6 | 8.2 |
| Multiple pulmonary nodules | 3 | 4.1 |
| Atelectasis | 3 | 4.1 |
| Nodule and atelectasis | 1 | 1.4 |
| Mass and atelectasis | 1 | 1.4 |
| Locations | ||
| The right upper lobe | 13 | 17.8 |
| The right middle lobe | 7 | 9.6 |
| The right lower lobe | 20 | 27.4 |
| The left upper lobe | 8 | 11 |
| The left lower lob | 20 | 27.4 |
| The left main bronchi | 3 | 4.1 |
| Bilateral | 2 | 2.7 |
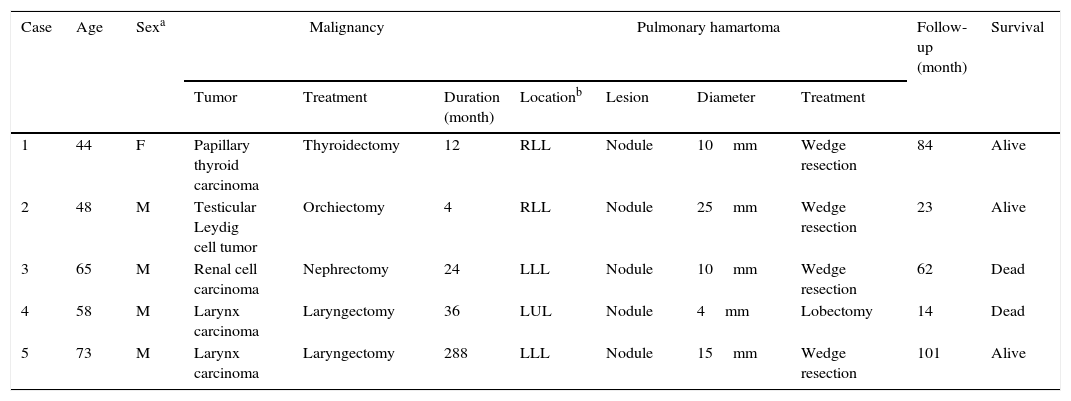
Twenty-three patients (24%) had malignancies, which occurred previously in five patients (Table 2), and concomitantly in 18 patients (Table 3). The patients with previous malignancies ranged in age from 44 to 73 years. While four male patients were smokers, one female patient had never smoked. Two patients (Case 1 and 2) were asymptomatic. The lesions were metachronous in four patients and synchronous in one patient. Radiologically, four patients had solitary pulmonary nodules with no findings associated with malignancy. One patient had a metastatic pulmonary nodule from renal cell carcinoma. FDG PET-CT was performed in four patients. In the patient with metastatic renal cell carcinoma, the SUVmax was 7.4 for the malignant lesion and ametabolic for the pulmonary hamartoma. These values were ametabolic, 1.8, and 3.4 in the remaining three patients, respectively.
Features of the hamartoma patients with previous malignancies.
| Case | Age | Sexa | Malignancy | Pulmonary hamartoma | Follow-up (month) | Survival | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | Treatment | Duration (month) | Locationb | Lesion | Diameter | Treatment | |||||
| 1 | 44 | F | Papillary thyroid carcinoma | Thyroidectomy | 12 | RLL | Nodule | 10mm | Wedge resection | 84 | Alive |
| 2 | 48 | M | Testicular Leydig cell tumor | Orchiectomy | 4 | RLL | Nodule | 25mm | Wedge resection | 23 | Alive |
| 3 | 65 | M | Renal cell carcinoma | Nephrectomy | 24 | LLL | Nodule | 10mm | Wedge resection | 62 | Dead |
| 4 | 58 | M | Larynx carcinoma | Laryngectomy | 36 | LUL | Nodule | 4mm | Lobectomy | 14 | Dead |
| 5 | 73 | M | Larynx carcinoma | Laryngectomy | 288 | LLL | Nodule | 15mm | Wedge resection | 101 | Alive |
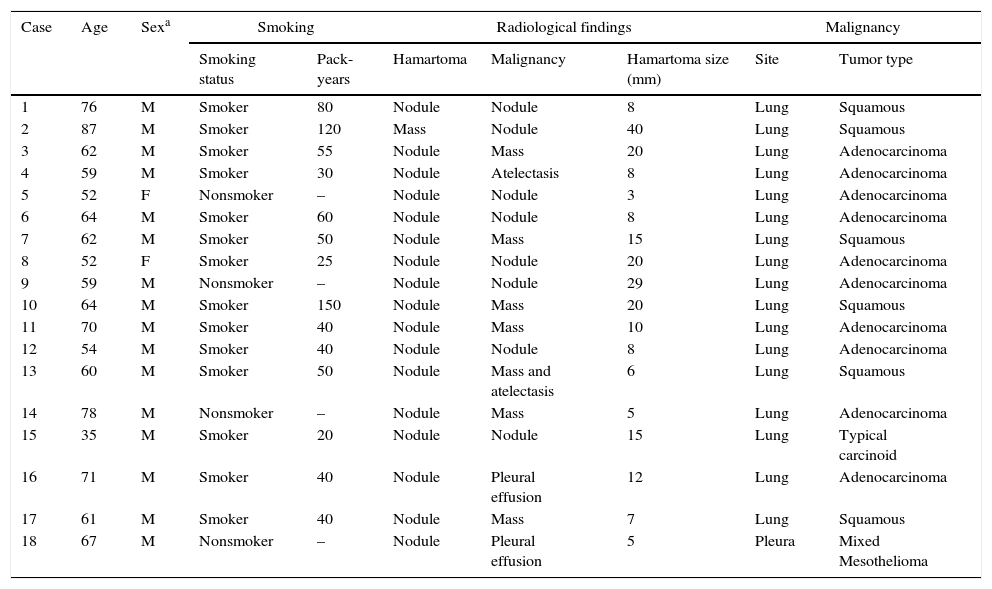
The clinical, radiological, and pathological features of the hamartoma patients with concomitant malignancies.
| Case | Age | Sexa | Smoking | Radiological findings | Malignancy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Smoking status | Pack-years | Hamartoma | Malignancy | Hamartoma size (mm) | Site | Tumor type | |||
| 1 | 76 | M | Smoker | 80 | Nodule | Nodule | 8 | Lung | Squamous |
| 2 | 87 | M | Smoker | 120 | Mass | Nodule | 40 | Lung | Squamous |
| 3 | 62 | M | Smoker | 55 | Nodule | Mass | 20 | Lung | Adenocarcinoma |
| 4 | 59 | M | Smoker | 30 | Nodule | Atelectasis | 8 | Lung | Adenocarcinoma |
| 5 | 52 | F | Nonsmoker | – | Nodule | Nodule | 3 | Lung | Adenocarcinoma |
| 6 | 64 | M | Smoker | 60 | Nodule | Nodule | 8 | Lung | Adenocarcinoma |
| 7 | 62 | M | Smoker | 50 | Nodule | Mass | 15 | Lung | Squamous |
| 8 | 52 | F | Smoker | 25 | Nodule | Nodule | 20 | Lung | Adenocarcinoma |
| 9 | 59 | M | Nonsmoker | – | Nodule | Nodule | 29 | Lung | Adenocarcinoma |
| 10 | 64 | M | Smoker | 150 | Nodule | Mass | 20 | Lung | Squamous |
| 11 | 70 | M | Smoker | 40 | Nodule | Mass | 10 | Lung | Adenocarcinoma |
| 12 | 54 | M | Smoker | 40 | Nodule | Nodule | 8 | Lung | Adenocarcinoma |
| 13 | 60 | M | Smoker | 50 | Nodule | Mass and atelectasis | 6 | Lung | Squamous |
| 14 | 78 | M | Nonsmoker | – | Nodule | Mass | 5 | Lung | Adenocarcinoma |
| 15 | 35 | M | Smoker | 20 | Nodule | Nodule | 15 | Lung | Typical carcinoid |
| 16 | 71 | M | Smoker | 40 | Nodule | Pleural effusion | 12 | Lung | Adenocarcinoma |
| 17 | 61 | M | Smoker | 40 | Nodule | Mass | 7 | Lung | Squamous |
| 18 | 67 | M | Nonsmoker | – | Nodule | Pleural effusion | 5 | Pleura | Mixed Mesothelioma |
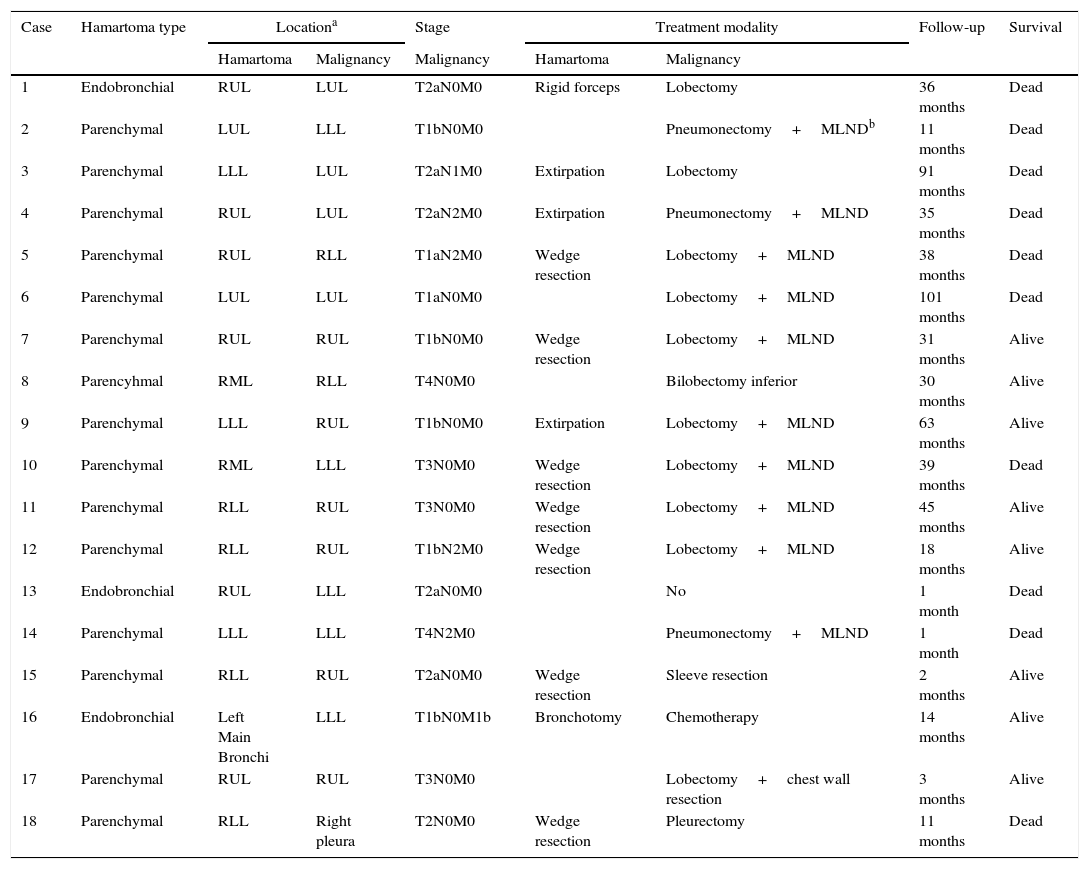
Stage, treatment, and outcome of the patients with concomitant malignancies.
| Case | Hamartoma type | Locationa | Stage | Treatment modality | Follow-up | Survival | ||
|---|---|---|---|---|---|---|---|---|
| Hamartoma | Malignancy | Malignancy | Hamartoma | Malignancy | ||||
| 1 | Endobronchial | RUL | LUL | T2aN0M0 | Rigid forceps | Lobectomy | 36 months | Dead |
| 2 | Parenchymal | LUL | LLL | T1bN0M0 | Pneumonectomy+MLNDb | 11 months | Dead | |
| 3 | Parenchymal | LLL | LUL | T2aN1M0 | Extirpation | Lobectomy | 91 months | Dead |
| 4 | Parenchymal | RUL | LUL | T2aN2M0 | Extirpation | Pneumonectomy+MLND | 35 months | Dead |
| 5 | Parenchymal | RUL | RLL | T1aN2M0 | Wedge resection | Lobectomy+MLND | 38 months | Dead |
| 6 | Parenchymal | LUL | LUL | T1aN0M0 | Lobectomy+MLND | 101 months | Dead | |
| 7 | Parenchymal | RUL | RUL | T1bN0M0 | Wedge resection | Lobectomy+MLND | 31 months | Alive |
| 8 | Parencyhmal | RML | RLL | T4N0M0 | Bilobectomy inferior | 30 months | Alive | |
| 9 | Parenchymal | LLL | RUL | T1bN0M0 | Extirpation | Lobectomy+MLND | 63 months | Alive |
| 10 | Parenchymal | RML | LLL | T3N0M0 | Wedge resection | Lobectomy+MLND | 39 months | Dead |
| 11 | Parenchymal | RLL | RUL | T3N0M0 | Wedge resection | Lobectomy+MLND | 45 months | Alive |
| 12 | Parenchymal | RLL | RUL | T1bN2M0 | Wedge resection | Lobectomy+MLND | 18 months | Alive |
| 13 | Endobronchial | RUL | LLL | T2aN0M0 | No | 1 month | Dead | |
| 14 | Parenchymal | LLL | LLL | T4N2M0 | Pneumonectomy+MLND | 1 month | Dead | |
| 15 | Parenchymal | RLL | RUL | T2aN0M0 | Wedge resection | Sleeve resection | 2 months | Alive |
| 16 | Endobronchial | Left Main Bronchi | LLL | T1bN0M1b | Bronchotomy | Chemotherapy | 14 months | Alive |
| 17 | Parenchymal | RUL | RUL | T3N0M0 | Lobectomy+chest wall resection | 3 months | Alive | |
| 18 | Parenchymal | RLL | Right pleura | T2N0M0 | Wedge resection | Pleurectomy | 11 months | Dead |
Of the 18 patients with concomitant malignancies, 16 were males and two were females, with a mean age of 63 years (range 35–87 years). Fourteen patients (77.8%) were smokers, with a mean duration of 57.1 pack-years (range 20–150 pack-years). Only one patient was symptom-free at presentation. Malignancies originated from the lung in 17 patients and from the pleura in one patient. Coexistence of a hamartoma and malignancy was diagnosed preoperatively in two patients. Malignancies were diagnosed before surgery and hamartomas after surgery in 10 patients. Preoperatively, five patients had no diagnoses of hamartoma or malignancy. One patient had the diagnosis of hamartoma before surgery, after which malignancy was diagnosed. Pulmonary hamartomas and malignancies were located in the ipsilateral lung in 12/17 patients and in the same lobe in four patients. FDG PET-CT was performed in 14 patients, all of whom had ametabolic SUVmax values for hamartomas. SUVmax values for malignancies ranged from 2.4 to 35.5, with a mean value of 14.4.
DiscussionBenign tumors of the lung are very rare, with pulmonary hamartoma being the most common.2 It accounts for 3% of all lung tumors and 77% of benign lung tumors.3,11 Pulmonary hamartomas can be parenchymal or endobronchial in location. The latter accounts for only 1–19.5% of pulmonary hamartomas.4 At presentation, most patients are free of symptoms, and pulmonary hamartomas are detected incidentally on chest X-rays obtained for other reasons. The typical radiographic feature is a coin lesion in the periphery of the lung. On rare occasions, pulmonary hamartomas can present as multiple nodules.10,12
Pulmonary hamartomas are generally considered to be benign neoplasms. However, there have been several reports on the association between hamartomas and malignancies,5–10,13,14 and the likelihood of malignant transformation to carcinoma or sarcoma.5–7 There are cases of adenocarcinoma, squamous cell carcinoma, and sarcoma arising from pulmonary hamartomas.7,13,14 Lee et al.14 reported a case of squamous cell carcinoma originating from a pulmonary hamartoma. Pulmonary hamartomas may also coexist with metastatic lung tumors. A literature review reported 10 cases of coexisting hamartomas and metastatic lung tumors.15 In addition, patients with pulmonary hamartomas can have synchronous or metachronous extrathoracic malignancies.16,17 The risk for lung cancer in pulmonary hamartoma patients was estimated to be 6.3 and 6.66 times as high as the age–sex–ethnicity adjusted rate expected for the general population.8,9 Pulmonary hamartomas can frequently be diagnosed in patients undergoing resection for primary lung cancers.18,19 Smith et al.18 found the prevalence of hamartoma to be 12% in patients undergoing resection for suspected lung cancers. Similarly, many patients with resected pulmonary hamartomas were found to have simultaneous, synchronous or metachronous lung cancers.2,9,10,20–22 Kawano et al.20 reported simultaneous primary lung cancers in 25% of patients with resected pulmonary hamartomas. Van den Bosch et al.10 found bronchial carcinomas in 11 of 154 patients with pulmonary hamartomas, synchronous in six and metachronous in five. It is clear that there is a high incidence of malignancies in cases with pulmonary hamartomas; however, the question as to whether these malignancies are coincidental entities or associated with malignant growth in existing hamartomas remains uncertain.2,7–10,20 Ruchita et al.7 found no pathophysiologic association between pulmonary hamartomas and lung cancer development. A previous report suggested an etiologic association between hamartomas and malignancies.8 Smoking and environmental carcinogenic factors have also been implicated as important risk factors for the development of lung carcinoma in patients with pulmonary hamartomas.2,9
We found concomitant malignancies in 23 patients (24%). Five of these had previous lesions. Radiologically, hamartomas in these patients presented as a solitary pulmonary nodule or multiple pulmonary nodules. When solitary or multiple pulmonary nodules are detected in patients with a known malignancy, authors tend to regard every nodule as a metastasis. However, these nodules can be associated with synchronous or metachronous primary lung cancers or benign lesions such as hamartoma, as seen in our cases.2,10,15,23 Malignancy was concomitant in 18 patients. Radiological examination showed multiple pulmonary lesions in all these patients. Most patients had a preoperative diagnosis of a malignant tumor and thus were evaluated for satellite lesions. When a satellite lesion is detected in a patient with known malignancy, we tend to consider it a metastatic lung tumor. However, these satellite lesions can be associated with simultaneous primary lung cancers or benign lesions accompanying malignant tumors.18,19,23 A previous report noted that the prevalence of a benign disease was 9% in patients undergoing resection for suspected lung cancers, with pulmonary hamartomas accounting for 9% of satellite benign lesions.18 When satellite nodules or masses are identified in patients with pulmonary hamartomas, we tend to consider them multiple pulmonary hamartomas, as was the case in our three patients. However, these lesions can be associated with other lung pathologies accompanying pulmonary hamartomas, including synchronous lung cancers, metastatic lung tumors, and benign pulmonary diseases such as tuberculosis.9,15,20 On the other hand, patients with pulmonary hamartomas may develop subsequent pulmonary lesions, which may be recurrences of pulmonary hamartomas or subsequent malignancies.2,21In the presence of a malignancy or hamartoma, it is important to make diagnostic investigations for the differential diagnosis of each pulmonary nodule.15,23
ConclusionsHamartomas are common and linked to the same exposures and risk factors as those for lung cancer, larynx cancer, and others. The majority of hamartomas are parenchymal and appear as a solitary pulmonary nodule on a roentgenogram. On rare occasions, pulmonary hamartomas can present as multiple nodules. Hamartomas may be accompanied by lung malignancies. The possibility of coexisting benign lesions such as hamartomas should be considered when solitary or multiple pulmonary lesions are found in patients with primary lung cancers or metastatic lung tumors.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestNone.