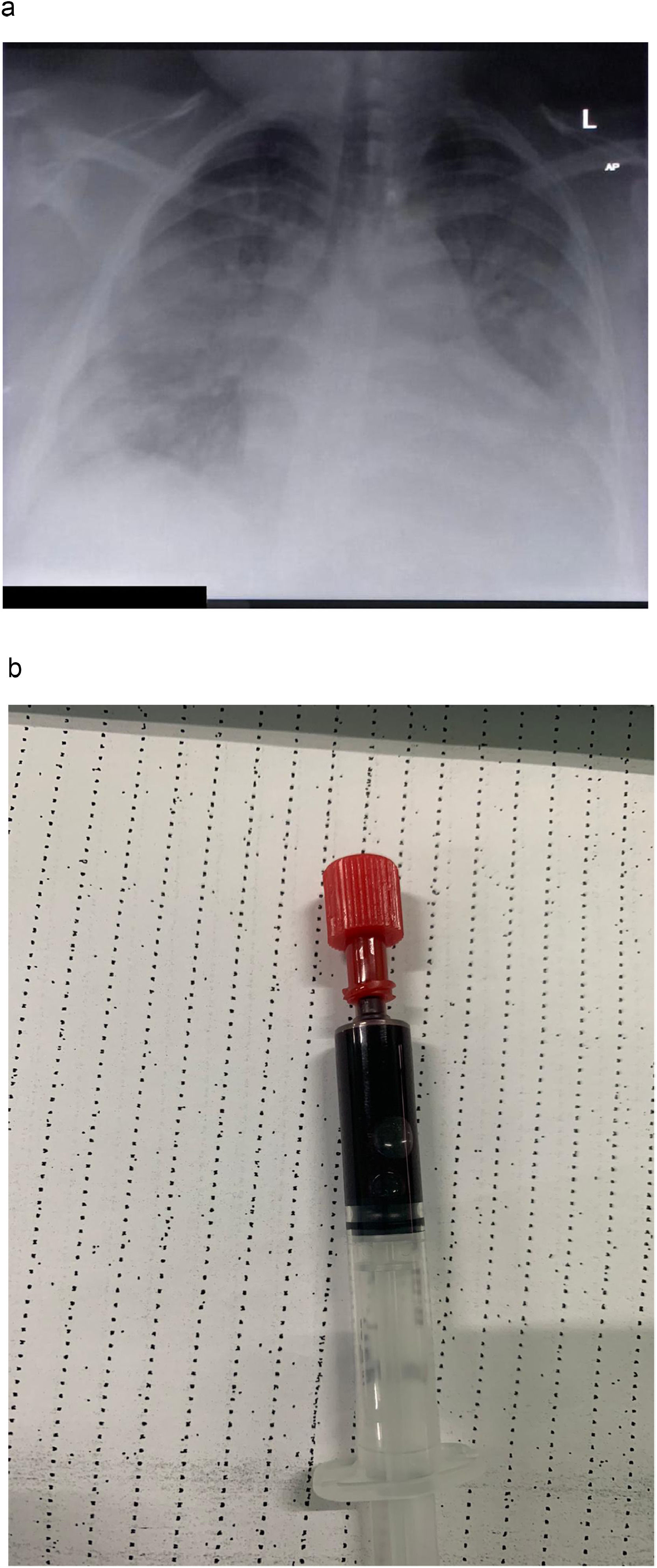
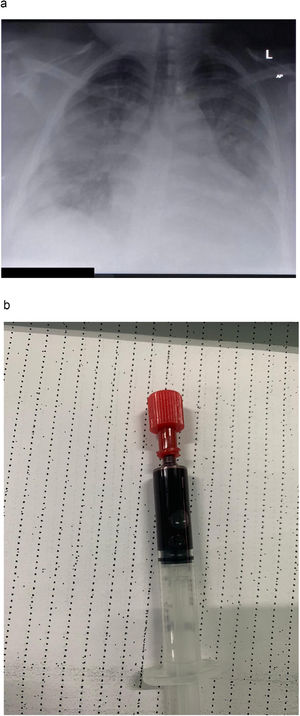
A 31 year-old female patient was admitted to our dedicated COVID-19 Intensive Care Unit with hypoxaemic respiratory insufficiency due to COVID-19 pneumonitis. Her past medical history was unremarkable, except for extreme obesity (BMI 70·3). She was not taking any medications, and was not under any regular medical care. Her chest X-ray at admission showed bilateral interstitial pulmonary consolidations (Fig. 1A). At admission, our patient was tachypneic, with a respiratory rate of 30-40/min, with SpO2 85% to 90% while on a non-rebreather mask. We started our patient on dexamethasone, tocilizumab and enoxaparin in a therapeutic dose. Oxygenation improved markedly upon initiation of non-invasive ventilation (NIV) through a mask interface (IPAP 12 cmH2O, EPAP 10 cmH2O, FiO2 started at 100% and titrated to SpO2). Initially, while on NIV, she maintained SpO2 > 90%, with FiO2 of 60-80%, whereas saturations would drop within minutes while on a non-rebreather mask (15L / min), during attempted breaks from NIV. We did not offer high-flow nasal cannula (HFNC) oxygen therapy, partially because of its excessively high oxygen consumption, which presented logistical barriers in our resource-limited setting during a pandemic. Furthermore, we expected “true” positive pressure ventilation to have more beneficial effects on obesity-related, position-dependent atelectasis, and on a potentially present obstructive sleep apnea. Over the first week of admission, she developed progressive hypoxaemia, while denying dyspnoea. Our patient repeatedly refused intubation and invasive ventilation, citing a lack of symptoms, and a fear for a worse outcome with mechanical ventilation. On day three, the patient desaturated to SpO2 of about 50% (despite FiO2100%), while denying dyspnoea, and without exhibiting tachypnea. Following prone positioning, saturations recovered to above 90%. Intermittent awake proning was, from that moment on, continued three times daily. On day seven, SpO2 again dropped to 30-55%, from then on never exceeding 60%, and at times reaching levels as low as 21%, with no further response to prone positioning. Changes in ventilatory settings (EPAP as low as 6 and up to 14cmH2O; as CPAP, or with pressure support of up to 6 cmH2O; FiO2 from here on never below 100%) yielded no improvement in respiratory parameters. An arterial line was placed in the left brachial artery, to allow for regular blood sampling. Arterial blood was extremely dark (Fig. 1B), with a PaO2 of 28mmHg. Accidental venous sampling was excluded with an adequate arterial blood pressure waveform. There was no methemoglobinaemia. Meanwhile, our patient was able to speak coherent, full sentences, and denied having any sensation of dyspnoea, although she was at times tachypneic. She remained firm in her view, and persistently refused endotracheal intubation. After enduring extreme hypoxaemia for about two days in a seemingly stable condition, she suddenly developed ventricular fibrillation, rapidly followed by asystole and death. Resuscitative efforts were not successful.
Chest X-ray taken in a patient with severe COVID-19. showing extensive bilateral opacities, with a slight predominance for the peripheral and basal lung fields. (B) Arterial blood sample, drawn from a patient with COVID-19 receiving non-invasive ventilation (EPAP 12; Pressure Support 4; FiO2 100%). Blood gas analysis revealed a PaO2 of 28mmHg (P/F ratio 28mmHg). Despite oxygen saturations below 40%, the patient denied dyspnoea and did not have elevated work of breathing.
Silent, or “happy” hypoxaemia is a well-known phenomenon in COVID-19.1,2 Several theories have been proposed for its existence, most revolving around intrapulmonary shunting as the primary driver of hypoxaemia, with relative preservation of lung compliance in the early stages of the disease,3 and resultant normocarbia or even hypocarbia,4 although a neural factor has also been proposed.5 In our case, we think a certain level of hypoxaemia at baseline associated with previously undiagnosed, but potentially present obesity hypoventilation syndrome may have contributed to our patient's tolerance of extremely low arterial oxygen levels.
The optimal timing of intubation in severe COVID-19 remains controversial. Early on in the pandemic, prominent authors urged clinicians to intubate early, in order to prevent patient self-induced lung injury (P-SILI), which was hypothesised to result from excessive respiratory effort.6 Furthermore, non-invasive respiratory support was feared to lead to droplet formation, putting health-care workers at risk.7 Conversely, it has been argued that early, potentially unnecessary intubation may increase mortality by exposing patients to the risks of sedation and invasive ventilation.8 As the pandemic progressed, many clinicians adopted a “wait-and-see” strategy, where patients are initially managed with non-invasive ventilatory support (including NIV and HFNC), and are intubated only upon failure of such therapies.9 More recent studies yield conflicting results, and although non-invasive respiratory support appears safe, and has been shown to reduce the need for invasive ventilation, the optimum timing of intubation in COVID-19 is as of yet still unknown.10 It may be reasonable to use a step-up approach, starting HFNC in patients who fail conventional oxygen therapy, failure of which could be followed by a trial of CPAP, and eventually, if indicated, intubation.11
To aid in deciding whom to intubate, authors have proposed using the ROX index,12 which, in several retrospective series, has been reported to predict failure of non-invasive respiratory support in COVID-19.12,13 While most studies were performed in cohorts treated with HFNC, the ROX index has been reported to correlate with outcomes after CPAP as well.14 It should be noted that different studies report different cut-offs (such as <3.8512 and <5.9913). Our patient had ROX-indexes as low as 1.12, clearly indicating a high risk of treatment failure.
The phenomenon of seemingly well-tolerated hypoxaemia in COVID-19 has led to further controversy, as it seems counter-intuitive to intubate a patient who, despite having low oxygen saturations, is feeling well. This discrepancy between subjective and objective findings has led some authors to argue that such patients should not be intubated, as long as they remain asymptomatic, and do not exhibit increased work of breathing.15 Even though we are inclined to agree with this concept in general, as long as hypoxaemia is mild to moderate, we believe our case demonstrates the dangers when such an approach is taken to the extreme. There is a point where hypoxaemia can lead to rapid cardiovascular decompensation,2 where “happy” hypoxaemia can show its unfriendly side: sudden cardiac death.
FundingThe authors declare that no external funding was received for the conduct of this study and/or the preparation of this manuscript.