Transbronchial lung cryobiopsy (TBC) has emerged as a diagnostic alternative to surgical lung biopsy in interstitial lung disease (ILD). Despite its less invasive nature, some associated complications have been described.
ObjectiveTo evaluate complications of TBC and associated factors.
MethodsProspective evaluation of all patients with ILD submitted to TBC in our centre. Clinicodemographic variables and factors associated to TBC complications were analyzed. The effect of the variables on the complication risk was evaluated by a logistic regression model.
ResultsNinety patients were included (mean age 60±13 years; 58.9% male). Twenty-two patients presented pneumothorax, 18 (81.8%) of which were treated with chest tube drainage [median air leak time: 1 day (IQR=2)]. Grade 2 and 3 bleeding was observed in 13 (14.4%) cases. Presence of visceral pleura in the sample accounted for almost more than 10 times the odds of pneumothorax (OR=9.59, 95% CI 2.95–31.17, p<0.001). Increased body mass index (BMI) was associated with bleeding (16% additional odds for each BMI unit increase (OR=1.16, 95% CI 1.01–1.34, p=0.049).
ConclusionThe most frequent complication of TBC was pneumothorax, although rapidly reversible. There was a positive association between pneumothorax and the presence of pleura in the biopsy samples as well as between bleeding and increased BMI. More studies about TBC complications are needed to improve the selection of the candidates for this procedure.
Interstitial lung disease (ILD) represents a heterogeneous group of diseases with different prognostic and therapeutic implications. The evaluation of patients with ILD is best achieved by a multidisciplinary approach combining clinical, radiologic, and pathologic features. In many circumstances a histological confirmation is required to establish the exact diagnosis.1–5 Several techniques have evolved over the years and are employed in many centres. Conventional transbronchial lung biopsy (TLB) is not currently recommended in fibrotic lung disorders due to its low diagnostic yield6; besides, the procedure presents risks.7
Surgical lung biopsy (SLB) provides adequate lung specimens for histopathological analysis and is recommended as reference standard by current guidelines in ILD when a non-invasive diagnosis cannot confidently be made.6,8 However, it requires hospitalization and chest tube drainage and may be associated with significant morbidity (2–20%), including prolonged air leak and acute exacerbation as well as mortality (2–17%).9–12 Moreover, many subjects are excluded because of increased risks of perioperative morbidity and mortality by a combination of advanced age, comorbidity, severe respiratory failure, and pulmonary hypertension.
Cryotherapy has been used in bronchoscopy since the sixties, mainly in the management of endobronchial lesions in patients with bronchial obstruction.13 More recently, transbronchial cryobiopsy (TBC) has been used for the assessment of ILD. The major advantage of TBC is that large pieces of tissue can be extracted with a higher percentage of alveolar tissue,14–18 fewer crush artefacts and less atelectasis.16–18 The greater volume of alveolar tissue correlates with a better diagnostic yield.15–19 Despite its less invasive nature, some associated complications have been described. The potential for bleeding and the pneumothorax rates up to 33%, often requiring inpatient admission and chest tube drainage, are the most commonly described.19–23
We here investigate the safety of TBC in subjects with ILD and possible factors associated with its complications, in order to improve candidate selection for this procedure.
MethodsStudy designWe conducted a prospective study of all patients with ILD undergoing TBC from May 2014 to December 2016 at the Bronchoscopy Unit of the Pulmonology Department of Vila Nova de Gaia/Espinho Hospital Centre. Medical records were analyzed and demographic data, chest high resolution computed tomographic (HRCT) scans, procedure and complications details and pathology were recorded. The indication for TBC was diffuse pulmonary infiltrates of unclear aetiology. The request for TBC was made by the pulmonologist dedicated to ILD. According to the standard of care protocol in our institution, patients were required to have a pO2 of at least 60mmHg under oxygen delivery up to 2liters/min, and normal blood count, electrolytes, markers of renal function and coagulation parameters. All anticoagulants were discontinued before the procedure as per guidelines.24 Before the procedure, risks and possible complications were explained to each patient and informed consent was obtained.
The procedureAll procedures were performed under general anaesthesia and by a pulmonologist experienced in interventional bronchoscopy. After intubation with a rigid tracheoscope (Storz 12mm®), a videobronchoscope was advanced to the desired segment (previously identified in the HRCT) and a flexible cryoprobe (2.4mm, model 20416-032, Erbokryo® CA, Erbe, Germany) was passed through the videobronchoscope into the bronchial segment under fluoroscopic guidance. After confirming correct positioning (defined as having the tip of the probe perpendicular and about to 10mm from the chest wall), a freezing time of 5s was applied, time after which the bronchoscope and cryoprobe were removed as a single unit and a bronchial blocker balloon (Olympus® B5-2c), previously placed in the segment was inflated, in order to prevent haemorrhage. The frozen specimen was thawed in saline and fixed in formalin. Each patient had at least one biopsy using this technique. Only one lung was biopsied per patient and the number of biopsies taken depended upon the researcher.
In patients who had not previous had bronchoalveolar lavage, it was performed before cryobiopsy, usually in the most affected pulmonary lobe.
ComplicationsSeverity of bleeding was classified as: grade 0 – no bleeding; grade 1 – estimated volume of aspirated blood <50ml; grade 2 – estimated volume of aspirated blood of between 50 and 100ml and requiring endoscopic procedures as instillation of topical adrenaline and/or ice-cold saline; grade 3 – estimated volume of aspirated blood >100ml and requiring endoscopic procedures as instillation of topical adrenaline and/or ice-cold saline and/or haemostatic tamponade therapy; grade 4 – any life-threatening bleeding requiring transfusion or escalation of care such as admission to the intensive care unit or surgery consultation. To rule out pneumothorax, a chest X-ray was obtained within at least 2h of the procedure.
Statistical analysisDescriptive statistics were used to analyze patient characteristics. The categorical variables were reported in frequencies (n) and percentages (%). Continuous data were described as means and standard deviations (SD) when variables had symmetric distributions and as median and interquartile range (IQR) when variables had no symmetric distribution. Inferences were tested by the chi-squared test (qualitative variables), the t-test (normally distributed quantitative variables) or the Mann–Whitney U-test (nonnormally distributed quantitative variables). The effect of the significant variables that emerged from the previous analysis on bleeding and pneumothorax risk was evaluated by a multivariate logistic regression model. A p value of <0.05 was considered significant. Data was analyzed using SPSS software version 22.
ResultsPatients and techniqueWe included 90 patients who underwent TBC. The procedure was performed mainly in ambulatory patients (n=66; 73.3%). Six patients (6.7%) were already hospitalized at our department, the majority of them with suspicious organizing pneumonia; 18 patients (20%) from other hospitals were previously admitted only for logistic reasons.
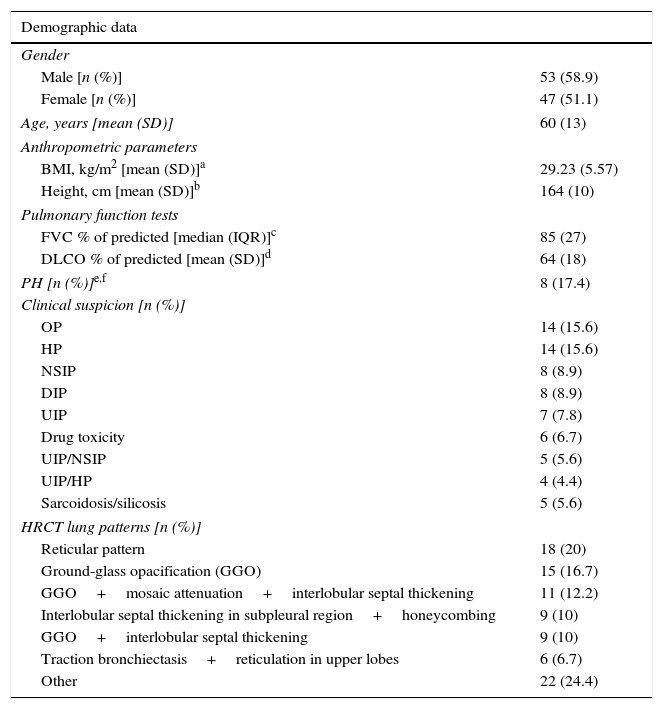
Baseline characteristics are represented in Table 1.
Patient characteristics.
| Demographic data | |
|---|---|
| Gender | |
| Male [n (%)] | 53 (58.9) |
| Female [n (%)] | 47 (51.1) |
| Age, years [mean (SD)] | 60 (13) |
| Anthropometric parameters | |
| BMI, kg/m2 [mean (SD)]a | 29.23 (5.57) |
| Height, cm [mean (SD)]b | 164 (10) |
| Pulmonary function tests | |
| FVC % of predicted [median (IQR)]c | 85 (27) |
| DLCO % of predicted [mean (SD)]d | 64 (18) |
| PH [n (%)]e,f | 8 (17.4) |
| Clinical suspicion [n (%)] | |
| OP | 14 (15.6) |
| HP | 14 (15.6) |
| NSIP | 8 (8.9) |
| DIP | 8 (8.9) |
| UIP | 7 (7.8) |
| Drug toxicity | 6 (6.7) |
| UIP/NSIP | 5 (5.6) |
| UIP/HP | 4 (4.4) |
| Sarcoidosis/silicosis | 5 (5.6) |
| HRCT lung patterns [n (%)] | |
| Reticular pattern | 18 (20) |
| Ground-glass opacification (GGO) | 15 (16.7) |
| GGO+mosaic attenuation+interlobular septal thickening | 11 (12.2) |
| Interlobular septal thickening in subpleural region+honeycombing | 9 (10) |
| GGO+interlobular septal thickening | 9 (10) |
| Traction bronchiectasis+reticulation in upper lobes | 6 (6.7) |
| Other | 22 (24.4) |
BMI – body max index; FVC – forced vital capacity; DLCO – diffusing capacity of the lung for carbon monoxide; PH – pulmonary hypertension; OP – organizing pneumonia; HP – hypersensitivity pneumonitis; NSIP – non-specific interstitial pneumonia; DIP – desquamative interstitial pneumonia; UIP – usual interstitial pneumonia
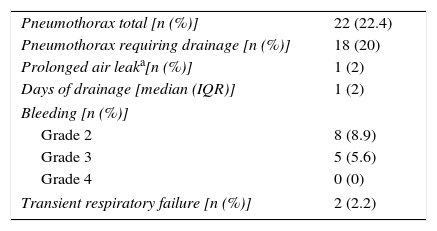
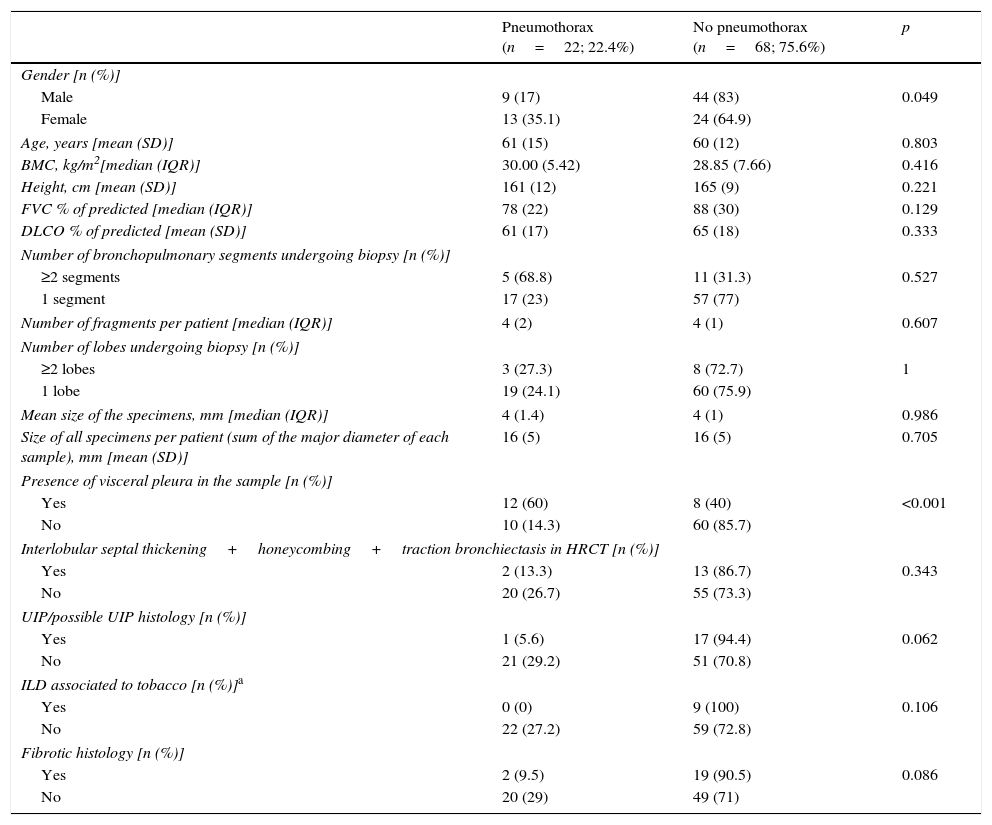
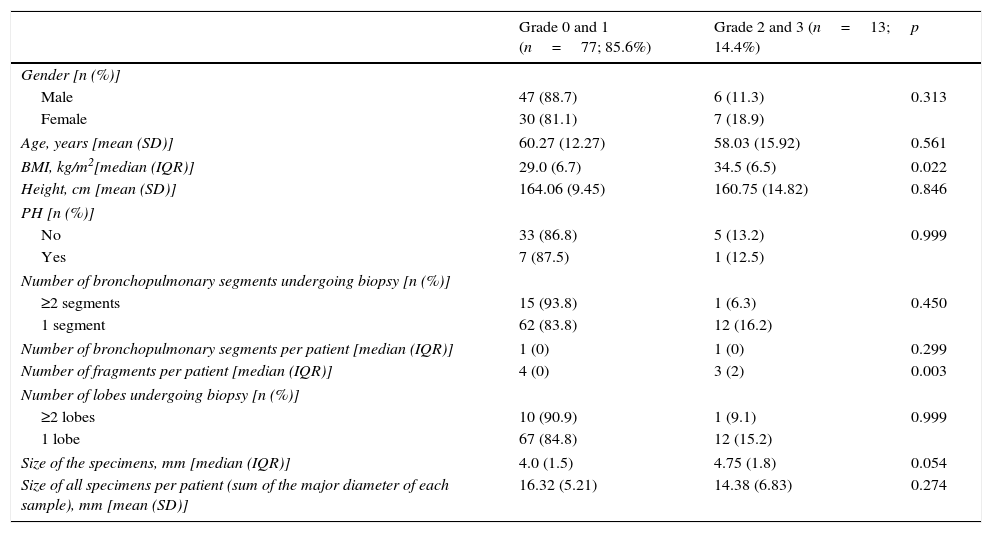
The main complications of TBC were pneumothorax and bleeding, as presented in Table 2.Pneumothorax was observed in 22 cases (22.4%), 18 of which (81.8% of total) required chest tube drainage. Median time of hospitalization was 1 day (IQR=2). One patient presented a prolonged in-hospital stay (20 days) because of a persistent air leak.The risk of pneumothorax was increased when fragments of pleura were present in the biopsy sample (p<0.001), but it did not correlate with number or size of specimens (p=0.607, p=0.986, respectively) (Table 3). A more detailed analysis was performed and we verified that in patients presenting fragments of pleura in the biopsy sample, the predicted DLCO% was lower in the group presenting pneumothorax (mean=54.71, SD=17.14) compared to the group without pneumothorax (mean=64.84, SD=18.62) (marginally significant, p=0.052). Female patients presented a higher risk of pneumothorax (p=0.049), but 88.9% of the female patients with pneumothorax presented visceral pleura in the biopsy samples (p<0.001). Considering histology we verified that in our series the occurrence of pneumothorax was not higher in the group of patients presenting UIP/possible UIP pattern or fibrotic patterns, although these results were only marginally significant (p=0.062 and p=0.086, respectively) (Table 3).Despite the preventive use of a bronchial blocker balloon during TBC techniques, grade 2 and 3 bleeding was encountered in 13 (14.4%) cases, 8 (8.9%) grade 2 and 5 (5.6%) grade 3 (Table 2). As shown in Table 4, there was a higher risk of grade 2 and 3 bleeding in patients presenting a higher BMI (p=0.022) but there was no major risk of bleeding related to height (p=0.846). We also addressed the issue of whether an increase in biopsies performed during each session of CBT and the mean size of the specimens raised the incidence of bleeding. There was no rise in the incidence of grade 2 and 3 bleeding when more cryobiopsies were performed in a session (p=0.003), although it should be noted that if the patients started to bleed the biopsy was not repeated; the risk of major bleeding tended to be higher with the increasing mean size of the samples (marginally significant, p=0.054) (Table 4). No patient required blood transfusion due to bleeding. Three patients (3.3%) required haemostatic tamponade therapy of a bronchial segment with oxidized regenerated cellulose due to bleeding, in the remaining patients no other measures beyond local treatment with cold saline or adrenaline was needed. All patients were discharged after bronchoscopy, and there was no recurrence of the haemoptysis.Considering pneumothorax and bleeding risk, a logistic regression analysis revealed that the presence of visceral pleura in the sample accounted for almost 10 times the odds of pneumothorax (OR=9.59, 95% CI 2.95–31.17, p<0.001). BMI accounted for 16% additional odds of bleeding for each BMI unit increase (OR=1.16, 95% CI 1.01–1.34, p=0.049).Two patients presented transient desaturation during the procedure with no need for interruption of the technique; both patients had a BMI over 30kg/m2.No other patients, besides the pneumothorax ones, needed hospitalization. We did not observe acute exacerbation of ILD, lung infection or mortality related to the procedure.
Adverse events.
| Pneumothorax total [n (%)] | 22 (22.4) |
| Pneumothorax requiring drainage [n (%)] | 18 (20) |
| Prolonged air leaka[n (%)] | 1 (2) |
| Days of drainage [median (IQR)] | 1 (2) |
| Bleeding [n (%)] | |
| Grade 2 | 8 (8.9) |
| Grade 3 | 5 (5.6) |
| Grade 4 | 0 (0) |
| Transient respiratory failure [n (%)] | 2 (2.2) |
Risk factors for pneumothorax.
| Pneumothorax (n=22; 22.4%) | No pneumothorax (n=68; 75.6%) | p | |
|---|---|---|---|
| Gender [n (%)] | |||
| Male | 9 (17) | 44 (83) | 0.049 |
| Female | 13 (35.1) | 24 (64.9) | |
| Age, years [mean (SD)] | 61 (15) | 60 (12) | 0.803 |
| BMC, kg/m2[median (IQR)] | 30.00 (5.42) | 28.85 (7.66) | 0.416 |
| Height, cm [mean (SD)] | 161 (12) | 165 (9) | 0.221 |
| FVC % of predicted [median (IQR)] | 78 (22) | 88 (30) | 0.129 |
| DLCO % of predicted [mean (SD)] | 61 (17) | 65 (18) | 0.333 |
| Number of bronchopulmonary segments undergoing biopsy [n (%)] | |||
| ≥2 segments | 5 (68.8) | 11 (31.3) | 0.527 |
| 1 segment | 17 (23) | 57 (77) | |
| Number of fragments per patient [median (IQR)] | 4 (2) | 4 (1) | 0.607 |
| Number of lobes undergoing biopsy [n (%)] | |||
| ≥2 lobes | 3 (27.3) | 8 (72.7) | 1 |
| 1 lobe | 19 (24.1) | 60 (75.9) | |
| Mean size of the specimens, mm [median (IQR)] | 4 (1.4) | 4 (1) | 0.986 |
| Size of all specimens per patient (sum of the major diameter of each sample), mm [mean (SD)] | 16 (5) | 16 (5) | 0.705 |
| Presence of visceral pleura in the sample [n (%)] | |||
| Yes | 12 (60) | 8 (40) | <0.001 |
| No | 10 (14.3) | 60 (85.7) | |
| Interlobular septal thickening+honeycombing+traction bronchiectasis in HRCT [n (%)] | |||
| Yes | 2 (13.3) | 13 (86.7) | 0.343 |
| No | 20 (26.7) | 55 (73.3) | |
| UIP/possible UIP histology [n (%)] | |||
| Yes | 1 (5.6) | 17 (94.4) | 0.062 |
| No | 21 (29.2) | 51 (70.8) | |
| ILD associated to tobacco [n (%)]a | |||
| Yes | 0 (0) | 9 (100) | 0.106 |
| No | 22 (27.2) | 59 (72.8) | |
| Fibrotic histology [n (%)] | |||
| Yes | 2 (9.5) | 19 (90.5) | 0.086 |
| No | 20 (29) | 49 (71) | |
Risk factors for bleeding.
| Grade 0 and 1 (n=77; 85.6%) | Grade 2 and 3 (n=13; 14.4%) | p | |
|---|---|---|---|
| Gender [n (%)] | |||
| Male | 47 (88.7) | 6 (11.3) | 0.313 |
| Female | 30 (81.1) | 7 (18.9) | |
| Age, years [mean (SD)] | 60.27 (12.27) | 58.03 (15.92) | 0.561 |
| BMI, kg/m2[median (IQR)] | 29.0 (6.7) | 34.5 (6.5) | 0.022 |
| Height, cm [mean (SD)] | 164.06 (9.45) | 160.75 (14.82) | 0.846 |
| PH [n (%)] | |||
| No | 33 (86.8) | 5 (13.2) | 0.999 |
| Yes | 7 (87.5) | 1 (12.5) | |
| Number of bronchopulmonary segments undergoing biopsy [n (%)] | |||
| ≥2 segments | 15 (93.8) | 1 (6.3) | 0.450 |
| 1 segment | 62 (83.8) | 12 (16.2) | |
| Number of bronchopulmonary segments per patient [median (IQR)] | 1 (0) | 1 (0) | 0.299 |
| Number of fragments per patient [median (IQR)] | 4 (0) | 3 (2) | 0.003 |
| Number of lobes undergoing biopsy [n (%)] | |||
| ≥2 lobes | 10 (90.9) | 1 (9.1) | 0.999 |
| 1 lobe | 67 (84.8) | 12 (15.2) | |
| Size of the specimens, mm [median (IQR)] | 4.0 (1.5) | 4.75 (1.8) | 0.054 |
| Size of all specimens per patient (sum of the major diameter of each sample), mm [mean (SD)] | 16.32 (5.21) | 14.38 (6.83) | 0.274 |
Obtaining tissue from lung parenchyma is often crucial for diagnosis and treatment in many ILD. Over the last few years TBC has been implemented as a mean of obtaining histological samples in the diagnostic setting of ILD; it has been used as a quite new modality and carried in some institutions. Few studies have explored its safety issues. We have here analyzed the TBC complications and its associated risk factors.
Among the adverse events, pneumothorax represented the most important complication of TBC with an incidence of 22.4% (22 of 90 patients; chest tube drainage was required in 18 cases (20%). The incidence of pneumothorax described in previous studies varies. Some studies reported no episodes of pneumothorax14,26–30 while others reported pneumothorax rates of between 1.4% and 12% (comparable to regular forceps biopsies or even lower).15,31–34 In a retrospective analysis that included 297 ILD patients undergoing cryobiopsy pneumothorax was the most common complication occurring in 60 patients (20.2%), 46 cases (15.5%) requiring drainage.21 Another study showed the occurrence of pneumothorax in 33% (19/58) patients undergoing TBC, with the majority (15/19) requiring chest tube drainage and hospitalization (average 6 days; range 1–9 days).23 In these studies the procedure was taken under spontaneous breathing and the probe was cooled for approximately 5–6s.
Hagmeyer et al. observed more pneumothorax events in patients submitted to jet ventilation (43%; 3/7) when compared to patients sedated and under spontaneous breathing over a bronchoscopy tube (12%; 3/25).22 In our study, all patients underwent general anaesthesia with jet ventilation; it could be a potential reason for the higher pneumothorax rate when compared to other studies that maintained spontaneous ventilation during the procedure. Also, the approximated time that the probe was cooled in our study was higher than the time used in other studies with lower advents frequencies. Furthermore, all our patients were intubated and that could also have played a role in increasing the pneumothorax risk. In a study by Polleti and Hetzel the authors analyzed the experience of previous studies with patients intubated and not intubated. They verified that in the ‘intubated group’ the mean size of samples was higher and there were more complications than in the ‘non-intubated group’.35
In a recent interesting study by Bango-Álvarez et al., TBC was performed under spontaneous breathing, under moderate sedation and without fluoroscopy; the cryoprobe was moved forward distally until it was noted that it could not advance further and then retracted 1–2cm. Using this technique they showed a low occurrence of pneumothorax (4.7%) and lack of pleura in all of the biopsies; which questions the need for fluoroscopy.34 On the other hand, we used fluoroscopy to guide the probe and defined a safe position as having its tip perpendicular and about 1cm from the chest wall. Our study population was limited to ILD patients; in the majority of these patients it is necessary to take peripheral subpleural biopsies in order to obtain samples that adequately represent the histopathologic processes, possibly explaining the high pneumothorax incidence.
We found that the risk of pneumothorax increases when fragments of pleura are present in the biopsy, similar to previous reports.19 In the study by Ravaglia et al. the degree of fibrosis of the lower lung zones was significantly increased in patients with pneumothorax compared to those without pneumothorax; furthermore, the authors also found a significant correlation between the incidence of pneumothorax and the histological UIP pattern.21 In our study we found opposite findings; only one patient with pneumothorax presented UIP/possible UIP pattern and only two patients presented fibrotic histology (comparing to other possible patterns), although with only a marginally statistical significance. This could be explained by the presumable additional caution in the execution of the technique because of the major risk of events in those patients.
It also must be noted that only a proportion of the patients would require chest tube drainage with TBC, compared with 100% patients who undergo surgical lung biopsy. In our sample, the pneumothoraces requiring drainage rapidly resolved; the median drainage time was 1 day, which was lower than the drainage time described in the previously referred studies. This result is important as chest tubes inserted for a prolonged time could be associated with an increased rate of infection.36
Although there is the potential for a large defect in the lung parenchyma following cryobiopsy, the occurrence of bleeding was low in our group (14.4%) and all cases were controlled only by local measures. In the majority of studies on this topic there were no episodes of severe bleeding needing blood transfusion or surgery and some studies did not report haemorrhage events15,30; other studies showed the occurrence of bleeding in up to 56.4%.16,17,22,29,31,33 The systematic review in the study by Ravaglia et al. reported no severe bleeding even in studies not employing the Fogarty balloon and moderate bleeding was documented in 7 out of 12 studies; however, the definition of ‘moderate’ bleeding was vastly divergent between the studies.21
The low incidence of significant bleeding in our cohort could be explained partly due to the preventive use of a bronchial blocker balloon, which is inflated immediately while the bronchoscope is being withdrawn from the airway to remove the biopsy specimen from the cryoprobe. Moreover, the haemostatic effects of cooling by cryo could probably have contributed to diminishing the occurrence of that event. However, the preventive use of a bronchial blocker balloon in our study is considered a ‘bias’ in the interpretation of the results as it does not allow us to quantify the true incidence of bleeding complications.
We found a positive association between the risk of bleeding and BMI that could be explained by the higher restriction and collapse of the peripheral lung areas in heavy patients that make it difficult for the probe to advance more peripherally. Thus the biopsies are done in more central areas increasing the bleeding risk.
Exacerbations of the ILD after the cryobiopsy are rare as well as associated deaths19,21 which occur less than in surgical lung biopsy.9–11 In our cohort, we did not have any acute exacerbation.
One of the major limitations of our analysis was the sample size. Although the collection of the data was prospective some data were missing. We also were not able to evaluate the influence of the greater experience of the bronchoscopist on the risks of complications.
In conclusion, pneumothorax was the most frequent complication, occurring in about a quarter of cases, and the presence of pleura in the biopsy was associated with its increased risk. Increased BMI seemed to be a risk factor for increased bleeding. The present analysis highlights the relative safety of the TBC procedure comparing to other possible approaches. Despite the obvious tendency to an increase in the use of this technique in different centres, there is still a lack of standardization of the procedure, as well as lack of defined indications and contra-indications, which would be of key importance not only to increase the diagnostic yield but also to reduce the potential complications of this technique. Another important issue is the grading of complications like bleeding, since its definition also differs among centres. More studies about TBC complications are needed in order to improve candidate selection for this procedure.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performedon humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in thisarticle.
Conflicts of interestThe authors have no conflicts of interest to declare.