The emergence of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) and its associated respiratory disease, coronavirus disease 2019 (COVID-19), has imposed social and medical burdens worldwide. Up to 12% of patients with SARS-CoV-2 infection required intensive care unit admission. Among them, 60-70% had acute hypoxic respiratory failure.1
High-flow nasal cannula (HFNC) oxygen therapy is the generally prescribed respiratory therapy for acute hypoxic respiratory failure. This therapy might help limit the need for invasive mechanical ventilation (IMV) and prevent the occurrence of associated adverse events such as ventilator-associated pneumonia in COVID-19 patients.2 However, administration of HFNC oxygen therapy in COVID-19 patients remains controversial, owing to uncertainties regarding the potential risk of viral transmission to healthcare workers, as this therapy is considered as an aerosol-generating procedure.3 Indeed, IMV can be selected when low-flow oxygen therapy through a nasal canula fails and a shortage of ventilators is a medical and social problem in regions particularly hard-hit by this pandemic. Therefore, a safe and effective respiratory management for COVID-19 patients should be urgently established.
Recent practical recommendations for COVID-19 patients indicate the use of a medical mask over the HFNC device to limit particle dispersion due to exhaled gas flow.4,5 These recommendations are partially supported by two previous experimental studies that indirectly examined exhaled breath by visualizing airflow movement using smoke6 and computational fluid dynamic (CFD) simulation.7 However, to the best of our knowledge, there is no direct evidence that this strategy could reduce the risk of SARS-CoV-2 transmission to healthcare workers in clinical settings due to the technical difficulty in direct visualization of particle dispersion. Here we present an experimental trial with a novel fine particle visualization system, which allowed evaluating whether particle dispersion from coughing while on HFNC oxygen could be suppressed by an appropriately placed medical mask.
We ran six scenarios with a healthy volunteer with nasal cannula at 3 L/min and 21% fraction of inspired oxygen (room air) delivered at 40 L/min 37℃ via HFNC (AIRVO™ 2 device with an Optiflow™ nasal interface [Fisher & Paykel, Auckland, New Zealand]). The volunteer was in a sitting position (seat height: 45 cm), and the evaluation was performed with and without wearing a standard medical mask. Particle dispersion was visualized by a video camera set at 29.97 frames per second (Eye Scope). This system used a light emitting diode (wavelength 400-410 nm; Parallel Eye D), which permitted a visualization of particle ≥1 μm in diameter. Images obtained were reconstructed as videos using commercial software (Particle Eye). Equipment described above depended on Shin Nippon Air Technologies (Tokyo, Japan).
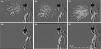
First, we identified exhaled particles dispersed from coughing in the absence of either nasal cannula, HFNC, or a mask, which reached a horizontal distance of 57 cm (Fig. 1A and supplemental video A). Second, exhaled particles were dispersed from coughing in a similar fashion when using nasal cannula (Fig. 1B and supplemental video B) and HFNC (Fig. 1C and supplemental video C), which reached a horizontal distance of 62 cm and 59 cm, respectively. Notably, when the volunteer wore a standard medical mask, no exhaled particles were detected from coughing either without (Fig. 1D and supplemental video D) or with concurrent nasal cannula (Fig. 1E and supplemental video E) or HFNC therapy (Fig. 1F and supplemental video F).
Photographs of droplet dispersion after cough when not utilizing (A) and utilizing supplemental oxygen via nasal cannula (B) and HFNC (C) and with an appropriately placed medical mask when not utilizing (D) and utilizing supplemental oxygen delivered by nasal cannula (E) and HFNC (F).
HFNC = high-flow nasal cannula.
This report substantially increases our understanding on the droplet dispersion risk during HFNC therapy. Wearing a medical mask over HFNC device almost completely suppressed particle dispersion induced by coughing. Our findings are first direct evidence that wearing a medical mask will be a useful manner in administering HFNC oxygen therapy and strongly support the recommendation as described above. Moreover, the present direct visualization of the suppressive effect of the medical mask in vivo further extends a previous CFD simulation by Leonard et al. who showed that the hypothetical medical mask captured 83.2% of particles (0.1-100 μm) during high-velocity nasal insufflation at 40 L/min.7
Despite the advanced technology, we and Leonard et al. could not visualize or simulate particles of <0.1 μm. Whether these small particles (aerosols) could be sources of transmission of SARS-CoV-2, remains unclear. Further studies are needed to evaluate whether HFNC oxygen therapy could increase risk of SARS-CoV-2 transmission to healthcare workers and whether wearing a medical mask under HFNC oxygen therapy could reduce this risk in clinical settings.
The visual evidence presented here should be shared with all care-givers wearing personal protective equipment to encourage the use of HFNC oxygen therapy for managing hypoxic COVID-19 patients. Hopefully, this method helps overcome this disastrous pandemic situation worldwide.
Conflict of interestSatoshi Hamada reports grants from Teijin Pharma, outside the submitted work.
Financial conflictsThis study was funded in part by the JSPS KAKENHI19K17634 (SH).
The Department of Advanced Medicine for Respiratory Failure is a Department of Collaborative Research Laboratory funded by Teijin Pharma.
The authors are grateful to Mr. Ryuta Okamoto, Mr. Taro Furukawa, and Mr. Kozo Takahashi, who are members of Shin Nippon Air Technologies, for technical assistance.