Prostacyclin (PGI2) has been shown to inhibit the expression of pro-inflammatory and pro-fibrotic mediators in pulmonary fibrosis. In this study, we aimed to test the preventive effects of intraperitoneally administered iloprost, a stable PGI2 analog, on bleomycin-induced pulmonary fibrosis in rats and to compare the effects of iloprost with the effects of methyl-prednisolone, a traditional therapy.
MethodsRats were randomly allocated into four groups: 1. Saline alone (n=6); 2. Bleomycin+placebo (n=7); 3. Bleomycin+methyl-prednisolone (n=7); 4. Bleomycin+iloprost (n=7). Fibrotic changes in the lungs were demonstrated by analyzing the cellular composition of bronchoalveolar lavage fluid, histological evaluation and lung hydroxyproline content.
ResultsFibrosis was made in the lungs of rats by bleomycin experimentally. Fibrosis scores in the methyl-prednisolone and the iloprost groups were significantly lower than in the placebo group (p<0.05). Furthermore, the score of the iloprost group was significantly lower than the score of the methyl-prednisolone group. The hydroxyproline content was significantly less in the methyl-prednisolone and the iloprost groups (p<0.05). In the placebo group, the neutrophil percentage in bronchoalveolar lavage was significantly higher than in the other groups, whereas the macrophage percentage in placebo group was significantly lower (p<0.05).
ConclusionIloprost has protective effect on the pulmonary fibrosis induced by bleomycin and it may be more effective in decreasing fibrotic changes than methyl-prednisolone.
A prostaciclina (PGI2) é conhecida por inibir a expressão de mediadores pró-inflamatórios e pró-fibróticos na fibrose pulmonar. Neste estudo, procurou-se testar os efeitos preventivos do iloprost administrado por via intraperitoneal, um análogo estável do PGI2, na fibrose pulmonar induzida por bleomicina em ratos e comparar os efeitos do iloprost com os efeitos da metil-prednisolona, uma terapia tradicional.
MétodosOs ratos foram divididos aleatoriamente em quatro grupos: 1. Apenas soro fisiológico (n=6); 2. Bleomicina + placebo (n=7); 3. Bleomicina + metil-prednisolona (n=7); 4. Bleomicina + iloprost (n=7). Foram demonstradas alterações fibróticas nos pulmões analisando a composição celular do líquido de lavagem bronco-alveolar, avaliação histológica e conteúdo de hidroxiprolina pulmonar.
ResultadosAparecimento de fibrose nos pulmões dos ratos tratados com bleomicina. Os resultados dos grupos tratados com metil-prednisolona e iloprost foram significativamente inferiores ao do grupo placebo (p<0.05). Além disso, o resultado do grupo de iloprost foi significativamente inferior ao resultado do grupo de metil-prednisolona. O teor de hidroxiprolina foi significativamente inferior nos grupos de metil-prednisolona e de iloprost (p <0.05). No grupo placebo, a percentagem de neutrófilos na lavagem bronco-alveolar foi significativamente mais elevada do que nos outros grupos, enquanto que a percentagem de macrófagos no grupo placebo foi significativamente inferior (p <0.05).
ConclusãoO Iloprost tem um efeito protetor sobre a fibrose pulmonar induzida por bleomicina e pode ser mais eficaz na diminuição de alterações fibróticas que a metil-prednisolona.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressively fatal lung disease characterized by fibroblast proliferation and extracellular matrix remodeling. Unfortunately, despite intensive investigations, the current pharmacologic therapy of IPF is limited and the results of therapy have been unsuccessful.1,2 Lung transplantation is considered as the only solution that has been shown to prolong survival; however, it can be applied to limited patient groups.3
Animal models play an important role in the investigation of IPF. Chronic diseases, such as IPF are more difficult to apply to a model because, etiology and natural history of IPF are unclear and there is no known single trigger able to induce “IPF” in animals.2 There are different models for inducing the pulmonary fibrosis. One of the models is intratracheal bleomycin (BLM) instillation. BLM is a chemotherapeutic antibiotic, and pulmonary fibrosis is one of the major adverse effects of this agent in human cancer therapy. Nowadays, the standard agent for inducing the experimental pulmonary fibrosis is BLM. BLM-induced pulmonary damage of animal lungs reflects histological and biochemical characteristics of fibrosis well. Meanwhile, there is an evidence that bioactive metabolites of arachidonic acid may regulate the fibroproliferative response in lung fibrosis.4,5 Deletion of 5-lypoxygenase (5-LO) leading to a deficiency in sulphidopeptide-leukotriene production ameliorated bleomycin-induced fibrosis in mice.6 Furthermore, antagonizing leukotriene (LT) B4 receptor (LT B4 is a metabolite synthesized by 5-LO) attenuated the lung fibrosis induced by BLM in mice by suppressing the production of inflammatory and fibrotic cytokines and by promoting the antifibrotic cytokine.7 In contrast to LTB4, prostanoids, which are metabolites of arachidonic acid are important regulators of pulmonary homeostasis. Prostacyclin inhibits fibroblast proliferation and collagen synthesis.8
PGI2, known as prostacyclin, is produced by the action of cyclooxygenase (COX)-2, as an antiproliferative molecule in the setting of BLM-induced pulmonary fibrosis.9 Lovgren et al. reported that mice lacking COX-2 derived PGI2 were susceptible to developing severe pulmonary fibrosis in response to BLM.10 In that case, it can be said that PGI2 may inhibit the development of pulmonary fibrosis. Therefore, Zhu et al. demonstrated that iloprost, a stable PGI2, prevents BLM-induced pulmonary fibrosis in a mouse model and these authors reported that prostacyclin may represent a novel pharmacological agent for treating pulmonary fibrosis.11 However, Dactor et al. reported that prostanoids protected against lung fibrosis when prostanoid was administered before bleomycin challenge but had no therapeutic effect when administered after bleomycin challenge.12
Corticosteroids suppress neutrophil and lymphocyte migration into the lung, decrease the level of immune complexes, and alter alveolar macrophages function but it is unclear that there is any survival advantage or prevention of fibrosis in patients treated with corticosteroids alone or in combination with other agents.3
This study aimed to investigate the effects of PGI2 by using iloprost on lung fibrosis induced by BLM exposure in a rat model and to compare the effects of iloprost with methyl-prednisolone, a traditional therapy.
Material and methodsExperimental animalsIn this study, 27 male Wistar albino rats, 12-week old, weighing 200–250g were purchased from Inonu University Experiment Animals Production and Research Center. All experiments were approved by the institutional committee of animal care. The rats were randomly allocated into 4 groups. 1. Saline alone (control group) (n=6); 2. BLM+placebo (n=7); 3. BLM+methyl-prednisolone (n=7); 4. BLM+iloprost (n=7).
BLM-induced lung fibrosisThe rats were anesthetized with 50mg/kg ketamin and 5mg/kg xyalazine intraperitoneally, followed by a single intratracheal injection of BLM hydrochloride (2.5mg/kg body weight in 0.25ml phosphate buffered saline (PBS), Nippon Kayaku, Japan) in 50ml of sterile saline. Control rats were injected the same volume of intratracheal saline instead of BLM. All the rats were killed 14 days after the intratracheal injection of bleomycin or PBS.
Experimental groupsIn control and placebo groups, injection of 0.1ml PBS started intraperitoneally two days before the intratracheal injection of PBS or BLM. This solution was administered intraperitoneally for 16 days in two groups. Steroid treated rats were injected 5mg/kg/day methyl-prednisolone (Mustafa Nevzat Ltd., Istanbul, Turkey) intraperitoneally two days before the BLM injection and drug was administered in the same dose for 16 days. In the iloprost group, iloprost (200μg/kg Schering, Berlimed, Spain) was dissolved in 500μl of PBS. The dose levels were based on a previous study in the literature.11 First doses of iloprost were administered intraperitoneally two days before the BLM injection. Iloprost was administered intraperitoneally for 16 days. Treatment procedures were based on previous drug studies in bleomycin-induced lung fibrosis in rats.2,11,13
Bronchoalveolar lavageOn day 14 after the BLM injection, rats were euthanized with xylazine (5mg/kg) and ketamine (50mg/kg) intraperitoneally. The trachea was cannulated by using a blunt needle attached to a syringe. Bronchoalveolar lavage (BAL) was performed four times with 0.8ml of PBS. Cell suspensions were concentrated by low speed centrifugation. The supernatant of BAL fluid was collected and kept at −70°C until used. The supernatant was used for the measurement of total cell counts. Total cells were counted on a hemocytometer and differential cell (alveolar macrophages, neutrophils, lymphocytes and eosinophils), counts were estimated from cystospine preparations by counting 300 cells stained with May-Grünwald-Giemsa.
Lung histopathologyAfter BAL, the left lungs of scarificed rats were removed, fixed in a buffered 10% formalin solution for 24h, embedded in parafin and sectioned at 5μm thickness. Ten consecutive longitudinal sections of the lungs were stained with hematoxylin & eosin (HE) and were examined for pulmonary fibrosis. For histopathological scoring of pulmonary fibrosis, each successive field was assessed using the semiquantitative grading system described by Ashcroft et al.14 The entire lung section was reviewed at a magnification of 100×. Thirty to thirty five fields in each section were analyzed and a score ranging from 0 (normal lung) to 8 (total fibrosis) was assigned. The mean score of all fields was taken as the fibrosis score of that lung section. Criteria for grading pulmonary fibrosis were as follows. Grade 0=normal lung; Grade 1=minimal fibrous thickening of alveolar or bronchial walls; Grade 2–3=moderate thickening of walls without obvious damage to lung architecture; Grade 4–5=increased fibrosis with definite damage to lung architecture and formation of fibrous bands or small fibrous mass; Grade 6–7=severe distortion of structure and large fibrous areas; Grade 8=total fibrotic obliteration of the field. The grading was independently performed for each rat in a blind manner by two pathologists.
Hydroxyproline assayAfter BAL, the right lungs were used for hydroxyproline content as an index of collagen accumulation. Lungs were homogenized in 1ml of PBS, and hydrolyzed by the addition of 1ml of 12N HCL at 120°C for 16h and dissolved in 2ml deionized water. Samples were incubated with Chloramine T solution for 20min at room temperature, and with Ehrlich's solution at 65°C for 15min. The absorbance of the Wnal reaction solutions at 550nm were measured and amount of hydroxyproline was obtained as microgram.15
Statistical analysisStatistics package program (SPSS 11.0 for Windows; SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. Data were expressed as mean±S.E.M. Comparison was made by one-way ANOVA followed by appropriate post hoc test including multiple comparison test (least significant difference). P value less than 0.05 was considered statistically significant.
ResultsInflammatory cells in airwaysBLM+placebo administration caused a significant increase in the percentage of the neutrophils in BAL fluid (21.1±2.7, p<0.05) (Table 1). The percentage of alveolar macrophages in BLM+placebo group was significantly lower (76.7±2.3, p<0.05). In rats treated with iloprost or methyl-prednisolone, the percentages of the neutrophil and alveolar macrophages remained similar to the levels of control rats. There were no significant differences in the percentages of lymphocytes and eosinophils among the four groups.
Effects of iloprost and methyl-prednisolone on bleomycin-induced changes in inflammatory cells in BAL fluid.
| Control(n=6) | BLM+placebo(n=7) | BLM+methyl-prednisolone(n=7) | BLM+iloprost(n=7) | |
|---|---|---|---|---|
| Macrophages | 91.6±0.6 | 76.7±2.3* | 91.5±1.8 | 91.8±2.0 |
| Neutrophils | 6.6±0.5 | 21.1±2.7* | 5.8±1.7 | 5.3±1.9 |
| Lymphocytes | 1.6±0.5 | 2.0±0.5 | 2.4±0.3 | 2.8±1.1 |
| Eosinophils | 0.0±0.0 | 0.1±0.1 | 0.1±0.1 | 0.0±0.0 |
Data presented as mean±SEM of groups.
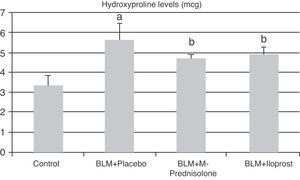
In the BLM+placebo, BLM administration produced a significant rise in lung tissue hyroxyproline content (5.65±0.78μg, p<0.05) as compared to those of the control group (3.37±0.51μg). The hydroxyproline content was significantly lower in BLM+methyl-prednisolone (4.72±0.25μg) and BLM+iloprost (4.90±0.39μg) groups (p<0.05) (Fig. 1). There was no significant difference in lung hyroxyproline content between BLM+iloprost and BLM+methyl-prednisolone groups.
The contents of hydroxyproline in the groups of control, BLM+placebo, BLM+methyl-prednisolone, BLM+iloprost. a The content of hydroxyproline in the BLM+placebo was significantly higher than the control group (p<0.05). b The hydroxyproline contents in the BLM+methyl-prednisolone and BLM+iloprost groups were significantly lower than the BLM+placebo groups (p<0.05).
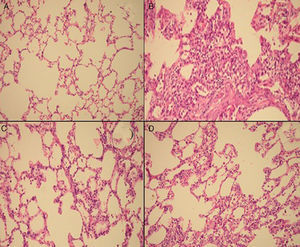
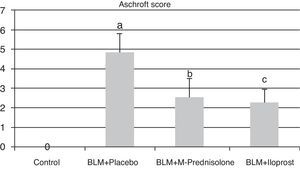
Semiquantitative assessment of the lung tissue section from the control group (with no treatment) revealed a normal alveolar structure (Fig. 2A). Fourteen days after BLM injection, rats administered BLM+placebo had more severe and extensive fibrosis (Fig. 2B). Alveolar walls of these rats were thickened and the air spaces were collapsed. Pulmonary fibrosis was less severe in the iloprost and methyl-prednisolone groups (Fig. 2C and D). Ashcroft score demonstrated that the degrees of pulmonary fibrosis in the BLM+methyl-prednisolone (2.57±0.94) and BLM+iloprost groups (2.29±0.68) were significantly lower than those of the BLM+placebo groups (4.86±0.93) (p<0.05) (Fig. 3). In the rats treated with iloprost, fibrosis score was significantly lower than those of the methyl-prednisolone group (p<0.05).
Fibrosis scores of the control, BLM+placebo, BLM+methyl-prednisolone and BLM+iloprost groups. a Ashcroft score in the BLM+placebo was significantly higher than the control group (p<0.05). b Ashcroft score in the BLM+methyl-prednisolone was significantly lower than the BLM+placebo groups (p<0.05). c Ashcroft score in the BLM+iloprost group was significantly lower than the BLM+methyl-prednisolone and BLM+placebo groups (p<0.05).
We assessed the effects of intraperitoneal administration of iloprost, a PGI2 analog, on BLM-induced pulmonary fibrosis and compared the effects of iloprost with methyl-prednisolone. Iloprost reduced BLM-induced lung fibrosis as shown by assessment of semiquantitative morphological indices of fibrosis and hydroxyproline content. Fibrosis score in iloprost group was significantly lower than in methyl-prednisolone group. Iloprost and methyl-prednisolone significantly attenuated lung infiltration by neutrophils. To the best of our knowledge, this is the second study of an intraperitoneal administration of iloprost in fibrosis induced by BLM in rats. The study of Zhu et al. is the first report of an intraperitoenal application of iloprost.11 However, our study is the first report to compare the preventive effects of iloprost with methyl-prednisolone on pulmonary fibrosis.
Hallmarks of pulmonary fibrosis include subepithelial fibroblastic foci and excessive deposition of collagen and extracellular matrix. Lung fibroblasts play an important role in the development of lung fibrosis.16 A number of studies have shown that prostanoids may play a role in limiting fibrotic responses in the lungs.5,8–11 Prostanoids are capable of inhibiting fibroblast migration, proliferation, and collagen synthesis. Patients with IPF, have been seen with a decrease of prostacyclin production from lung fibroblasts.17 Prostacyclin syntase expression has been demonstrated on pneumocytes, fibroblasts and endothelial cells in the healthy lungs. However, this expression did not continue to increase after induction of fibrosis with BLM.10 The treatment of rats with prostacyclin provided protection against BLM-induced fibrosis. Our data showed that fibrosis score and hyroxyproline levels decreased in rats treated with iloprost and methyl-prednisolone groups.
This point implies that neutrophils were markedly augmented in lungs of rats exposed to BLM+placebo and the augmentation of neutrophils into the lung was inhibited by BLM+iloprost and BLM+methyl-prednisolone. In rats treated with iloprost and methyl-prednisolone, alveolar macrophages remained similar to the levels of control rats whereas alveolar macrophages in BLM+placebo group were significantly lower. Zhu et al. showed that the number of inflammatory cells and lymphocytes accumulated in lungs was significantly higher in the BLM (no iloprost) group than those treated with iloprost. However, there was no significant difference in the number of macrophages and neutrophils in BAL fluid between the BLM (no iloprost) and the BLM+iloprost groups.11 These divergent results may be due to differences in study protocol, since Zhu et al. only administered iloprost 10–15min prior to injection of BLM. Bleomycin increases both neutrophils and lymphocytes in the lungs. However, in our study, lymphocytes did not significantly accumulate in the lungs in the BLM+placebo group. In rats treated with iloprost and methyl-prednisolone, lymphocytes remained similar to the levels of BLM+placebo group.
During the last 50 years, corticosteroid use has evolved into accepted practice in the treatment of IPF despite the fact that no prospective randomised placebo-controlled trial has ever been performed.3 Transient clinical response was found in a small minority of patients with no survival benefit compared to untreated patients in some studies.17–21 There has been a small but statistically significant reduction in the overall use of corticosteroids as classic treatment since the publication of treatment guidelines in recent years.22 Richeldi et al., carried out a systematic review of the efficacy of corticosteroids in the treatment of IPF reporting that no randomized controlled trial or clinical control trials in which corticosteroid treatment was compared to placebo were available. According to this review, results did not support the efficacy of corticosteroid in the treatment of IPF.23 The development of new IPF pathogenetic paradigms has driven research into new therapeutic strategies.24 In literature, there are some human studies which have evaluated the therapeutic effects in severe pulmonary hypertension secondary to pulmonary fibrosis.24,25 However, there is no human study in English literature which compares the therapeutic effects and superiority of prostacyclin analogs with corticosteroids in patients with IPF. Animal studies show that prostacyclin may have an important protective role in fibrotic disease5,10,11 but in these studies, the effects of prostacyclin analogs were not compared with traditional therapies such as corticosteroids. In the study of Zhu et al., iloprost was given 10–15min prior to intratracheal BLM. The results of this study indicated that iloprost could be preventive, but possibly not therapeutic for fibrosis.11 The authors recommended additional studies to evaluate both the reversal effect of iloprost in a time- and dose-dependent fashion and whether long-term treatment with iloprost would be beneficial for patients with fibrotic lung diseases. In the study of Dactor et al., mice were administered a single dose of bleomycin via oropharyngeal aspiration. Prostaglandin E2 and iloprost were administered 7 days before or 14 days after bleomycin challenge. When administered 7 days before bleomycin challenge, prostaglandin E2 protected against lung fibrosis. However, this agent had no therapeutic effect on lung fibrosis when administered 14 days after bleomycin.12 In our study, first doses of iloprost were administered two days before the BLM injection and it was continued intraperitoneally at the same doses for 16 days. Intraperitoneal administration of iloprost attenuated the development of BLM-induced pulmonary fibrosis and iloprost significantly improved fibrotic process in comparison to methyl-prednisolone.
In this present study, we did not compare groups according to survival and mortality which was expected up to the time of evaluation. No side-effects related to iloprost therapy were observed.
In conclusion, our findings provide evidence of the beneficial effects of iloprost in decreasing pulmonary fibrosis induced by BLM, namely by decreased inflammation, lung damage and fibrogenic activity in lung tissue.
Conflicts of interestThe authors have no conflicts of interest to declare.