Good's Syndrome is a rare cause of immunodeficiency associated with thymoma. Patients with this syndrome are prone to infections with encapsulated microorganisms. The diagnosis may be delayed for a considerable time period even after the thymectomy.
Case presentationWe describe the case of a 70-year-old woman with a background of thymectomy who presented with pneumonia and gram negative sepsis. Haemophilus influenzae was found in blood cultures. Moreover, there was evidence of impaired B and T cell immunity consistent with Good's Syndrome. She was commenced on immunoglobulin replacement following treatment of sepsis and remains well 18 months after the initial presentation.
ConclusionThis case illustrates the importance of considering Good's Syndrome in the context of pneumonia and immunodeficiency associated with encapsulated organisms such as Haemophilus influenzae. This clinical entity is associated with a significant mortality and should be considered as a cause of immunodeficiency even years after thymectomy.
A Síndrome de Good é uma causa rara da imunodeficiência associada ao timoma. Os pacientes com esta síndrome são propensos a infecções por microrganismos encapsulados. O diagnóstico pode ser atrasado por bastante tempo, mesmo após a timectomia.
Apresentação do CasoDescrevemos o caso de uma mulher de 70 anos com antecedentes de timectomia, que apresentava pneumonia e sepsis por agente gram negativo. O Haemophilus influenzae foi isolado em hemoculturas. Além disso, evidência de alterações da imunidade celular B e T, consistente com a Síndrome de Good. A doente iniciou terapêutica de substituição com imunoglobulina seguida de tratamento da sepsis e continua bem 18 meses após a apresentação inicial.
ConclusãoEste caso ilustra a importância de considerar a Síndrome de Good no contexto da pneumonia e imunodeficiência associadas a organismos encapsulados, como Haemophilus influenzae. Esta entidade clínica está associada a uma mortalidade significativa e deve ser considerada como uma causa de imunodeficiência mesmo anos depois da timectomia.
A 70-year-old woman presented to our medical assessment unit with a 4-week history of breathlessness, productive cough, weight loss and lethargy. She was a lifelong non-smoker. There was no history of chest pain, wheeze or hemoptysis. She had been recently treated with amoxicillin for a lower respiratory tract infection. Her past medical history included a minimally invasive type AB thymoma surgically resected 2 years previously. She had not received any chemo-radiotherapy. She had been thoroughly investigated 1 year previously with colonoscopy and abdominal/pelvic CT scan for chronic diarrhea of 2 years’ duration. However, no abnormalities were found. There was no past history of recurrent infections and there was no family history of immunodeficiency. However, there was evidence of immunoglobulin reduction (IgA 0.42g/L, IgM 0.20g/L and normal IgG) at the time of thymic resection.
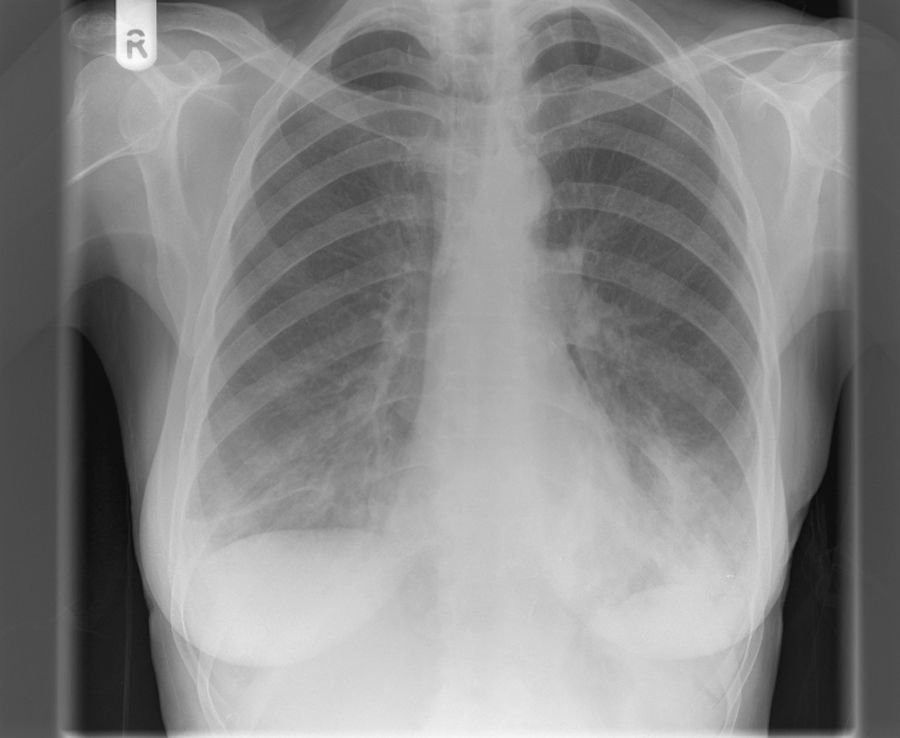
On examination, she was apyrexial but tachycardic with PR 117/min and BP 104/68mmHg. There was no clubbing or lymphadenopathy. There were bilateral basal crackles on chest examination. The remainder of the examination was unremarkable. Oxygen saturations were 88% on air with type 1 respiratory failure while receiving 28% oxygen [Arterial blood gas (pO2 6.5kPa, pCO2 5kPa, pH 7.50, HCO3 26mmol/L)]. Initial laboratory tests showed mild anaemia with Hb 10.4g/dl, WCC 4.5×109/L and platelets 187×109/L. The differential count showed lymphopenia of 0.38×109/L (normal range 1.5–3.5). She had mild hyponatraemia with Na 131mmol/L. C-reactive protein was markedly elevated to 295mg/L. Chest radiograph on admission revealed bilateral basal consolidation (Fig. 1). She was commenced on treatment for community acquired pneumonia with co-amoxiclav and clarithromycin. Blood cultures showed a growth of gram negative bacilli, Haemophilus influenzae, which was sensitive to all common antimicrobial agents. Subsequent sputum culture revealed growth of Pseudomonas aeruginosa and Candida albicans. Screening for HIV and CMV IgM was negative. A thoracic CT scan confirmed radiographic findings of bilateral basal consolidation with minimal pleural effusions, without any evidence of malignant disease in lung parenchyma or pleura.
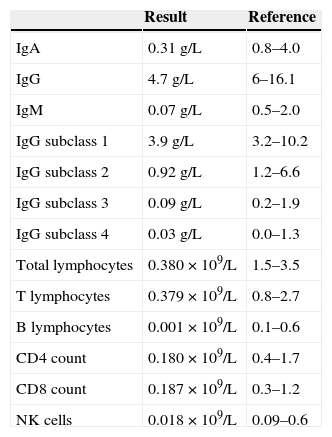
In view of the lymphopenia, lymphocyte subsets were obtained which showed that B cells were absent and markedly reduced T lymphocytes and natural killer (NK) cells (Table 1). Moreover, there was panhypogammaglobulinemia on immunoglobulin analysis. A full autoimmunity study was done which was negative. These findings of combined B and T cell deficiency in the context of previous thymoma suggested the diagnosis of Good's Syndrome. She responded well to antimicrobials and supportive care. Her functional antibody responses were found to be abnormal [Tetanus IgG 0.230mg/L (protective level >2.5mg/L), Pneumococcal IgG 46.9mg/L (protective level >30mg/L) and Haemophilus IgG 0.610mg/L (protective level >1mg/L)]. She was commenced on immunoglobulin replacement therapy to prevent further severe infective episodes in future. At 18-month follow up she is currently well.
Immunoglobulin and lymphocyte subset analysis.
| Result | Reference | |
|---|---|---|
| IgA | 0.31g/L | 0.8–4.0 |
| IgG | 4.7g/L | 6–16.1 |
| IgM | 0.07g/L | 0.5–2.0 |
| IgG subclass 1 | 3.9g/L | 3.2–10.2 |
| IgG subclass 2 | 0.92g/L | 1.2–6.6 |
| IgG subclass 3 | 0.09g/L | 0.2–1.9 |
| IgG subclass 4 | 0.03g/L | 0.0–1.3 |
| Total lymphocytes | 0.380×109/L | 1.5–3.5 |
| T lymphocytes | 0.379×109/L | 0.8–2.7 |
| B lymphocytes | 0.001×109/L | 0.1–0.6 |
| CD4 count | 0.180×109/L | 0.4–1.7 |
| CD8 count | 0.187×109/L | 0.3–1.2 |
| NK cells | 0.018×109/L | 0.09–0.6 |
Good's Syndrome is a rare cause of combined B and T cell immunodeficiency which predisposes the individual to bacterial infections with encapsulated organisms as well as opportunistic fungal and viral infections. It was originally described by Dr Robert Good1 who described a case of hypogammaglobulinemia associated with thymoma. The exact cause and pathogenesis of this syndrome is unknown, however it is suggested that it is a bone marrow defect associated with pre-B cell lymphopenia.2 Moreover, there is also evidence of frequent co-existence of eosinopenia3 with this syndrome.
The clinical features of this disorder are variable. It usually presents in the 4th or 5th decade of life with symptoms due to thymoma, including cough, dysphagia, dyspnea or hoarseness of voice or related to associated infections (as noted in the case presented), the most common being recurrent sinopulmonary infection secondary to encapsulated organisms.4 As described in our case, about 50% of patients with Good's Syndrome develop diarrhea which might be secondary to inflammatory colitis seen in patients with common variable immunodeficiency (CVID), or idiopathic as no pathogens are isolated in the majority of patients. Furthermore, CMV colitis may be a potential cause of increased bowel frequency and an infectious cause of diarrhea including enteric bacteria, CMV and giardia should be excluded as part of the investigation in patients with Good's Syndrome.
In terms of laboratory findings, anaemia is commonly associated with immunodeficiency and is seen in up to 50% of patients. The cause of anemia may well be pure red cell aplasia,5 pernicious anemia6 or hemolytic anemia.7 The most common micro-organisms associated with Good's Syndrome are encapsulated bacteria. Tarr and co workers reported that Haemophilus influenzae was grown in 24% and Streptococcus pneumoniae was isolated in 8% of their analyses of 51 cases of Good's Syndrome. The most common viral infection associated with this syndrome is cytomegalovirus. Moreover, herpes simplex and varicella zoster may be isolated in some cases.
The predominant immunological findings in Good's Syndrome are hypogammaglobulinemia, reduced/absent B cells and CD4+ T cell lymphopenia. The pathogenesis of the immunoglobulin deficiency is not fully understood. Oritani and colleagues8 have shown that limitin, an interferon like cytokine, can inhibit B cell growth and differentiation. Moreover, there is evidence that thymus itself can influence precursor B cell growth and maturation.9 A unique immunological finding in this case was a profound deficiency of NK cells and it would be interesting to investigate and monitor this aspect to gain further insight into this disorder.
The management of Good's Syndrome includes surgical resection of the thymoma. The most significant indicator of prognosis is the completeness of surgical resection. Advanced stage tumors may need combination radiotherapy with or without chemotherapy. The histology is usually a spindle cell variant but epithelial and mixed tumors have been noted as well. Medical management involves prompt treatment of infection, identification and management of concomitant bronchiectasis and immunoglobulin replacement. It is important to note that the surgical removal of thymoma does not reverse the immunoglobulin deficiency as evidenced by this case where the patient presented 2 years after her surgery. Furthermore, immunoglobulin replacement therapy is shown to reduce the incidence of infections including sinopulmonary infections.4 Functional antibody responses to Tetanus toxoid, Haemophilus B and Pneumococcal capsular polysaccharide antigen should be tested prior to the commencement of immunoglobulin replacement.
The prognosis of Good's Syndrome is worse than X linked agammaglobulinaemia and CVID10 and mortality of approximately 45% has been reported in a systematic review of 152 patients with this syndrome.11 The predominant cause of death is infection associated with immunodeficiency and it is important to recognize and treat infectious complications in this disease to improve mortality.
In conclusion, this case illustrates the importance of considering the possibility of Good's Syndrome in patients with gram negative bacteraemia and chronic diarrhea in the context of immunodeficiency. Moreover, it highlights the importance of evaluating immune status even years after the diagnosis of thymoma. In appropriate clinical context, Good's Syndrome should be suspected despite normal immune status at the time of the diagnosis of thymoma. Close collaboration between immunologists, microbiologists and physicians is invaluable for appropriate management of this rare disease of combined B and T cell immunodeficiency.