The incidence of iatrogenic pneumothorax (IPx) will increase with invasive procedures particularly at training hospitals, that is why we have made a retrospective study of the common diagnostic or therapeutic causes of IPx and its impact on morbidity. From January 2011 to December 2011, 36 patients developed IPx as emergencies, after an invasive procedure. Their mean age was 38 years (range: 19–69 years). Of the patients, 21 (58%) were male and 15 (42%) were female. The purpose was diagnostic in 6 cases and therapeutic in 30 cases. In 8 patients (22%) the procedure was performed due to underlying lung diseases and in 28 patients (78%) for other diseases. The procedure most frequently causing IPnx was central venous catheterization, with 20 patients (55%), other frequent causes were mechanical ventilation in 8 cases (22%) (of whom we reported 3 cases of bilateral pneumothorax), 6 cases of thoracentesis (16%) and 2 patients had life-saving percutaneous tracheotomy. The majority of our patients were managed by a small chest tube placement (unilateral n=30, bilateral n=3). The average duration of drainage was 3 days (range: 1–15 days), sadly one of our patients died of ischemic brain damage 15 days after tracheotomy.
At training hospitals the incidence of IPnx will increase with the increase in invasive procedures, which should only be performed by experienced personnel or under their supervision.
A incidência de pneumotórax iatrogénico (IPx) vai aumentar com procedimentos invasivos particularmente em hospitais de formação, sendo esse o motivo pelo qual fizemos um estudo retrospetivo do diagnóstico ou das causas terapêuticas comuns de IPx e do seu impacto na morbilidade. Desde janeiro de 2011 até dezembro de 2011, 36 pacientes desenvolveram IPx como emergências, depois de um procedimento invasivo. A sua média de idades foi de 38 anos (intervalo: 19-69 anos). Dos pacientes, 21 (58%) eram do sexo masculino e 15 (42%) do sexo feminino. O objetivo era diagnóstico em 6 casos e terapêutico em 30 casos. Em 8 pacientes (22%) o procedimento foi realizado devido a doenças pulmonares subjacentes e em 28 pacientes (78%) por outras doenças. O procedimento que mais frequentemente provocou IPnx foi a cateterização venosa central, com 20 doentes (55%), outras causas frequentes foram a ventilação mecânica, 8 casos (22%) dos quais foram relatados 3 casos de pneumotórax bilateral, 6 casos de toracocentese (16%) e 2 pacientes traqueotomia percutânea de socorro. A maioria dos nossos pacientes foram submetidos à colocação de um pequeno dreno torácico (unilateral n=30, bilateral n=3). A duração média da drenagem foi de 3 dias (intervalo: 1-15 dias), tendo infelizmente um dos nossos pacientes falecido devido a dano cerebral isquémico, 15 dias após a traqueotomia.
Em hospitais de formação a incidência de IPnx aumentará com o cada vez maior número de procedimentos invasivos, que apenas devem ser desempenhados por pessoal experiente ou sob a supervisão do mesmo.
Pneumothorax is a serious but common complication of invasive chest procedures. Several procedures were identified as causing pneumothorax, but according to the literature the most frequent causes are thoracocentesis, central venous cannulation and barotraumas.
In the present climate of increased use of invasive procedures, the incidence of iatrogenic pneumothorax (IPx) is going to increase particularly in training hospitals. It is for this reason that we want to assess and delineate retrospectively the common diagnostic or therapeutic causes of IPx and its impact on morbidity.
Materials and methodsFrom January 2011 to December 2011, we listed 36 cases of patients who developed IPx in the emergency department, after an invasive procedure for diagnostic or therapeutic purposes. For each patient details of age, gender, the invasive procedure which caused IPnx, the specific treatment and consequences were recorded.
ResultsThe mean age was 38 years (range: 19–69 years). Twenty-one (58%) of the patients were male and 15 (42%) were female. All the invasive procedures which caused IPnx were performed as emergencies in the Emergency Department. The purpose of the invasive procedure was diagnostic in 6 cases and therapeutic in 30 cases.
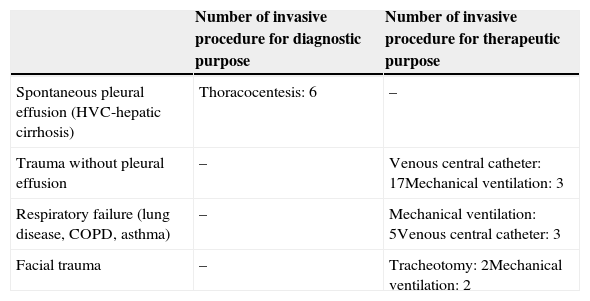
8 patients (22%) had procedures for underlying lung or tracheal diseases and 28 patients (78%) for other diseases (Table 1).
diseases associated with the iatrogenous pneumothorax.
| Number of invasive procedure for diagnostic purpose | Number of invasive procedure for therapeutic purpose | |
|---|---|---|
| Spontaneous pleural effusion (HVC-hepatic cirrhosis) | Thoracocentesis: 6 | – |
| Trauma without pleural effusion | – | Venous central catheter: 17Mechanical ventilation: 3 |
| Respiratory failure (lung disease, COPD, asthma) | – | Mechanical ventilation: 5Venous central catheter: 3 |
| Facial trauma | – | Tracheotomy: 2Mechanical ventilation: 2 |
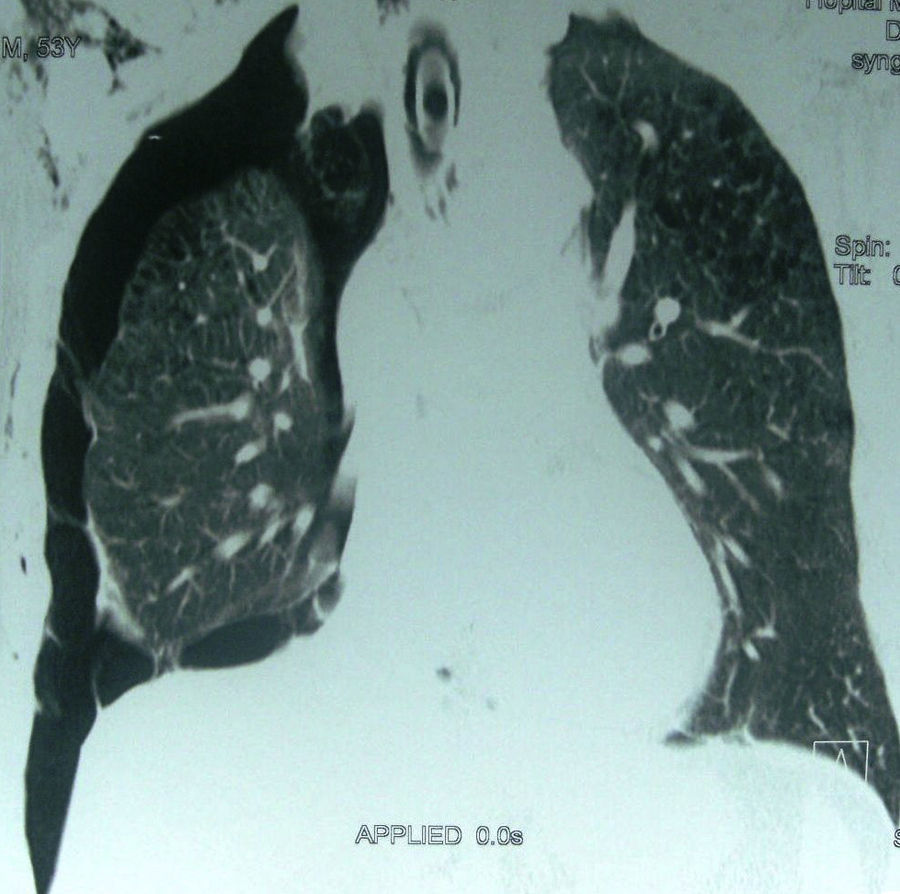
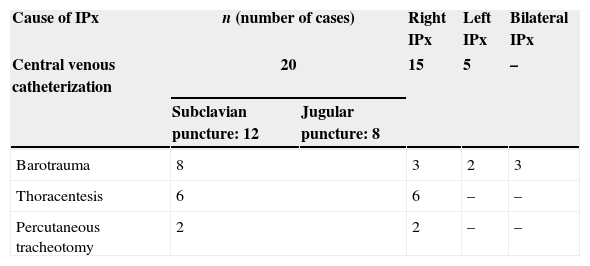
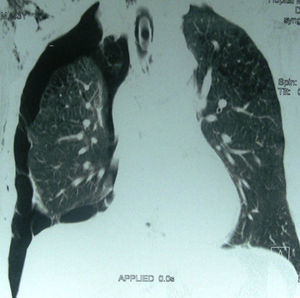
The procedure type most frequently causing IPnx was subclavian and jugular central venous catheterization; 20 patients (55%) (right side: n=15; left side: n=5). The other frequent causes were barotrauma due to mechanical ventilation (Fig. 1): 8 cases (22%). Of this 8 there were 3 cases with bilateral pneumothorax, 6 cases of thoracentesis (16%) and 2 patients had life-saving percutaneous tracheotomy (Table 2).
Clinical signs compatible with a pneumothorax were found in all patients with associated radiographic signs.
Percutaneous tracheotomies were performed by the physician (not a surgeon) who admitted the patient and indicated a life saving tracheotomy in the intensive care unit.
According to our protocol, once the invasive procedure is accomplished a systematic chest radiograph is taken.
Regardless of the causal procedure, and depending on patient stability and the size of the pneumothorax the majority (n=33, 91.6%) of our patients were managed by a small chest tube placement (unilateral n=30, bilateral n=3).
The chest tube placement was performed by a trained physician, using either the ventral approach (2–3 Intercostals space) in the mid-clavicular line, also called the approach according to Monaldi (n=8, chest tube Joly size 16), or the lateral approach (4–6 Intercostal space) in the anterior to mid-axillary line, also called the approach according to Bulau (n=28, chest tube Joly size 16–18). The tube is connected to a bottle or canister. Suction is often used to help it drain. Otherwise, gravity alone will allow it to drain.
Once the drain was in place, a chest radiograph was taken to check the location of the drain. The tube stayed in for as long as there was air or fluid to be removed, or the risk of air gathering.
Clinical malfunction of the tube with a radiological malposition was noted in 6 cases with a chest tube replacement by a larger size chest tube (Joly size 18). Malfunction was due to the interlobar placement of the tube.
The average of duration of drainage was 3 days (range: 1–15 days); sadly one of our patients died of ischemic brain damage 15 days after percutaneous tracheotomy.
Hospitalization was prolonged due to this treatment in only 10 cases, presumably because of the underlying disease which required long hospitalization.
DiscussionTo the best of our knowledge Iatrogenous Pneumothorax has very rarely been reported in the English literature; we entered “pneumothorax” AND “Iatrogenous” in Pubmed and there were no retrospective or prospective studies about the incidence of this complication which is one of the most common complications of invasive chest procedures. The morbidity associated with pneumothorax, particularly with a chest tube placement, should be a warning to physicians to avoid all unnecessary pneumothorax-related procedures especially in emergencies.
Careful choice of the site for percutaneous central vascular access is recommended, and the internal jugular vein is preferable because of the lower frequency of related pneumothorax, 7 IPx of 424 subclavian puncture site in the study of Pikwer,1 and 4 IPx of 1552 jugular puncture in the same study. Eisen et al.2 obtained similar results and concluded that a higher risk of pneumothorax was associated with the subclavian puncture, in our study 12 of the 20 patients had a subclavian approach, which is explained by anatomical proximity of sub clavian vessels to the pleural space and the greater familiarity of physicians with this site but Schummer et al.3 noted in a prospective study of 1794 catheterizations similar risk of IPx in subclavian and internal jugular site.
Thoracocentesis in order to evacuate recurrent or large pleural effusions is traditionally a safe procedure when performed by an experienced operator. IPx occurred in 19% in the study of Grogan et al.,4 and 5.1% in the recent study of Henry et al.5 However the latter found no statistically significant association between the occurrence of pneumothorax and the type of needle used, the size of the effusion, the amount of fluid drained, the presence of loculations, the type of thoracentesis, or the experience of the operator. All our thoracocentesis-related pneumothoraces were performed by training operators so we conclude that thoracocentesis can be difficult and needs a controlled setting.
In our study, we did not use ultrasound control after six thoracocentesis but a chest radiograph control. However, several studies have demonstrated high sensitivity and specificity for thoracic ultrasound in detection of occult pneumothorax in critical care,6 and trauma patients.7 The absence of lung sliding suggests pneumothorax, but it can occur in multiple other conditions such as mainstem intubation, acute respiratory distress syndrome, or pleural adhesions. The “lung point” is an ultrasound sign with 100% specificity for pneumothorax,8 and can be used to determine the size of the pneumothorax.9
Very few studies have been published about the role of tracheotomy in the pathogenesis of pneumothorax. Glicklick et al.10 reported 17% IPx in his earlier study of 45 tracheotomies. Pneumothorax is more significantly possible when tracheotomy is an emergency 11. Some authors11 proved experimentally the mechanism of pneumothorax via a pneumomediastinum secondary to air way hyper pressure during tracheotomy and surgery.
Complications with mechanical ventilation are common, and rupture of the pulmonary alveoli or airways after a sudden increase of intra-alveolar pressure is a common cause of pneumothorax. The incidence of pneumothorax during ARDS is less than 10% since the reduction of tidal volume and limiting plateau pressure. The more aggressive use of ventilator pressure characteristics was found to be associated with a higher incidence of pneumothorax, 17% in the study of Miller12 who noted the major role of protective lung strategy. In our study, the 8 cases with barotrauma had an associated Acute Respiratory Distress Syndrome and lung obstructive disease which increased the side effects of mechanical ventilation. An existing lung disease such as Chronic Obstructive Pulmonary Disease (COPD), or asthma is the most common cause of a greater associated incidence of pneumothorax after positive pressure ventilation.13 These patients have generally bleb or bulla due to airway obstruction. Acute respiratory distress syndrome (ARDS) is defined by bilateral pulmonary infiltrates with a decreased lung compliance responsible for high alveolar pressure even using low tidal volume. Chest X-ray or Computed Tomography is used to detect the air leak: pneumothorax or pneumomediastinum with possible risk of haemodynamic complications. Risk factors associated with barotrauma are the underlying disease for mechanical ventilation and a high airway pressure during mechanical ventilation.14 Alveolar pressure, obtained by measuring plateau pressure at the end of the inspiration, is the best tool for evaluating the risk of alveolar barotrauma. This pressure must be kept below 30cm H2O.14
Barotraumatic pneumothorax was managed by chest tube placement to prevent tension pneumothorax and haemodynamic complications and in our cases the time the chest tube was in place was longer (4 days). We use a preventive protocol in mechanic ventilation to avoid alveolar hyperpressure depending on the underlying lung disease and severity of the ARDS: a controlled low tidal volume ventilation is taken.
It is commonly believed that the size of a pneumothorax is an important determinant of treatment decision particularly as to whether chest tube drainage is required. In our practice we still use clinical and chest radiological data to take a decision; however, a volumetric quantification of pneumothoraces has been performed in a Chinese study15 by automated computer-aided volumetry in CT images. The results indicated that the inclusion of volume made it the most statistically significant indicators compared to the other tests in which volume was excluded from the clinical parameters.15 This study provides the evidence for the application of volumetric quantification of pneumothoraces in the management of clinically stable chest trauma patients with traumatic pneumothorax but it is based on computed tomography images which are not a systematic part of our practice.
Most authors recommend the lateral approach for chest tube insertion.16,17 without data documenting why they advise against using the ventral approach. To the best of our knowledge there are no studies which just examine the ventral approach or investigate differences between the two common approaches. Some authors recommend that if an approach other than the lateral is chosen then it should be performed by a thoracic surgeon.18,19
The ATLS manual recommends that the chest tube should “usually” be inserted via the lateral approach.18 In our protocol, to avoid the potential risk of lacerating the internal mammary artery and to have a safer intercostals space for preparation we use the lateral approach which is frequently described as a “safe zone”. We reserve the ventral approach for anterior pneumothoraces.
ConclusionThis review summarizes the current state of etiologies of IPx. Another factor is that we had many attending physicians on-scene. The influence of differing individual skills and different levels of familiarity with invasive procedures can affect the incidence of iatrogenous complications. So it is advisable to use ultrasound guidance whenever possible for central venous cannulation or thoracocentesis. If pneumothorax occurs, it is important to recognize the signs and symptoms particularly during mechanical ventilation or with underlying lung disease. A chest X-ray should be promptly done. Depending on its size and symptoms, treatment can vary from simple observation to a chest tube placement to be performed by experienced personnel or under their supervision even at training hospitals.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.