There is convincing evidence that obstructive sleep apnea (OSA) is highly associated with impaired glucose metabolism.
ObjectivesAnalyze the prevalence of OSA in type 1 and type 2 diabetes mellitus (DM) patients. Evaluate the influence of OSA on glycemic control.
MethodsThe adult patients with diabetes mellitus (DM) followed in the department of internal medicine were referred to our Sleep Unit. A home respiratory polygraphy was then performed on all patients with body mass index (BMI) < 40 kg/m2. The glycemic control was assessed by the value of glycated hemoglobin (Hba1c) in the previous 3 months.
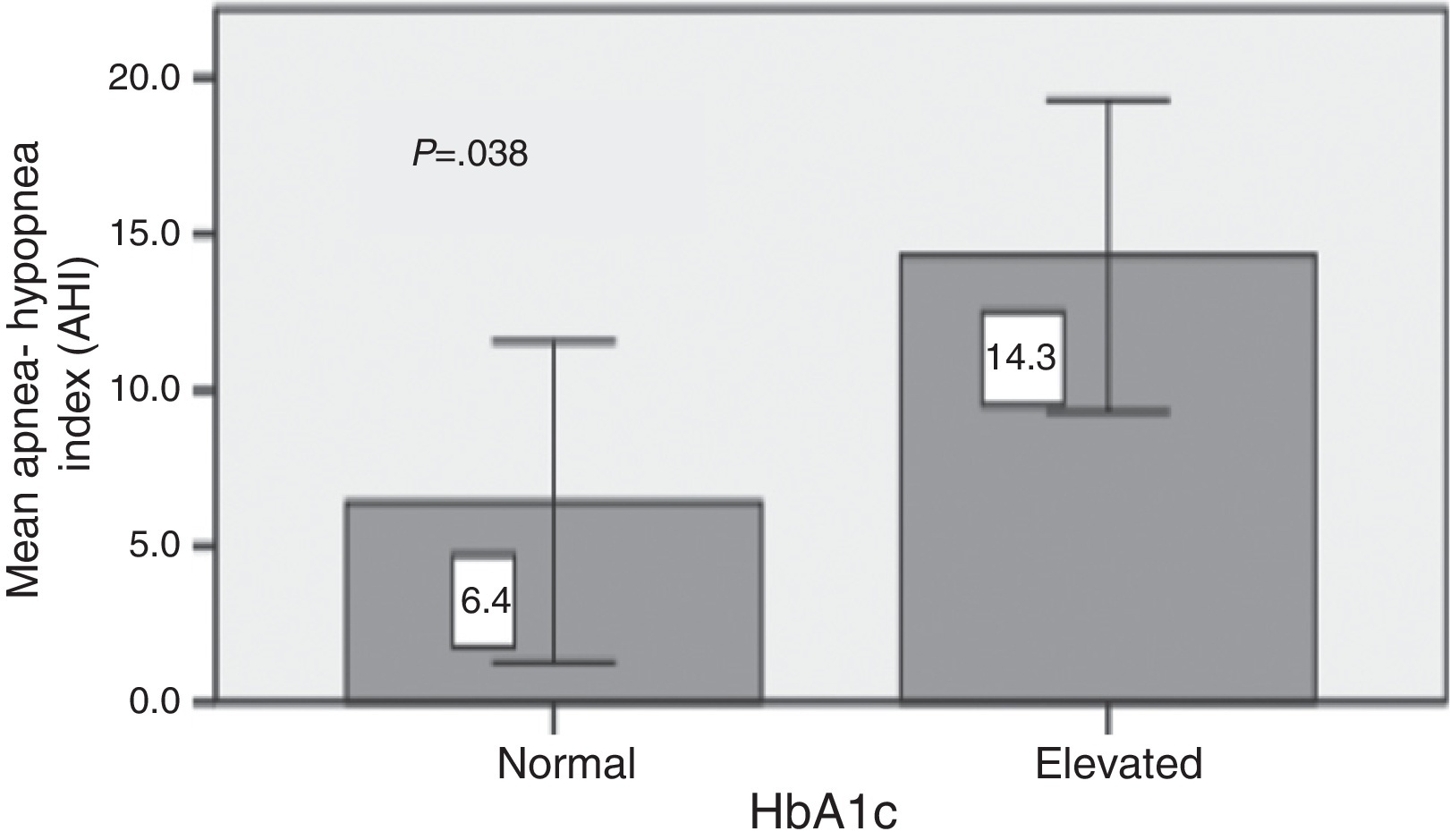
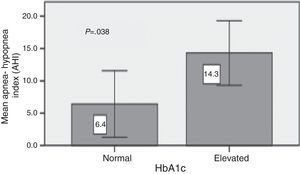
ResultsA total of 46 patients were studied (20 men and 26 women), the mean age was 50 ± 15 years and mean BMI was 28.6 ± 4.9 kg/m2. The mean Hba1c was 8.3 ± 1.2. Twenty three patients had type 2 DM and 23 patients had type 1 DM. Twenty nine patients (63.0%) had OSA and 8.7% had severe OSA (AHI > 30/h). The mean CT90 was 5.3 ± 12.5 and the mean AHI was 13.6 ± 18.3. The mean AHI was similar between type 1 and type 2 DM (15.7 ± 24.5 Vs 11.6 ± 8.9; p = 0.46). The AHI was not correlated with the BMI. Type 2 DM patients with poor glycemic control (HbA1c > 7.5%) had a significantly higher mean AHI (14.3 ± 9.0 vs 6.4 ± 6.2; p = 0.038). This difference did not remain significant after adjustment for BMI (p = 0.151).
ConclusionsThe prevalence of OSA in type 1 DM is similar to that found in type 2 DM. We note the high prevalence of OSA in younger patients with type 1 DM.
Obstructive sleep apnea (OSA) is a treatable sleep disorder characterized by repetitive upper airway collapse, leading to oxygen desaturation and sleep fragmentation.1, 2 Diabetes mellitus (DM) and obstructive sleep apnea (OSA) are common disorders that often coexist. One explanation for this overlap is the presence of shared risk factors such as obesity. There may also be a more complex relationship between these conditions in which an underlying metabolic disorder predisposes for both or in which metabolic and autonomic abnormalities associated with one influence the development of the other. Because both diabetes and OSA are associated with increased cardiovascular morbidity and mortality, it is possible that the presence of both conditions results in added or even synergistic health risks. OSA has been identified as a highly prevalent comorbidity of type 2 diabetes mellitus (DM),3, 4, 5 in particular, among obese patients with type 2 DM, which represent the vast majority of individuals with type 2 DM. Multiple epidemiologic and clinical studies have revealed that individuals without diabetes suffering from OSA show alterations in glucose metabolism, including insulin resistance and impaired glucose tolerance, independent of adiposity.6, 7, 8, 9
Previous studies on the relationship between sleep characteristics and diabetes have mainly focused on patients with type 2 diabetes. Only a few studies have assessed sleep characteristics in patients with type 1 diabetes mellitus. Those studies investigated mainly children with type 1 diabetes, with shorter duration of diabetes. Previous studies showed that reduction of sleep duration and/or decreased sleep quality impair glucose tolerance and reduce insulin sensitivity in healthy controls.10 Sleep disturbances might have a similar negative effect on glucose metabolism in patients with type 1 diabetes, resulting in worse glucose control.11 Borel et al.12 observed a prevalence of 40% in 37 non-obese adult patients with type 1 diabetes mellitus.
In the present study, we therefore evaluated the prevalence of OSA in type 1 and type 2 diabetes mellitus (DM) patients and the influence of OSA on glycemic control.
MethodsWe included patients (over 18 years old and under 80) with diabetes followed in the Diabetes Unity of our Hospital, from January 2012 to December 2013. All participants had been on stable medications for diabetes and other comorbidities for the preceding 3 months. Subjects were excluded if they: had unstable cardiopulmonary, neurological, or psychiatric disease; morbid obesity (BMI ≥ 40 kg/m2); or used nocturnal oxygen or positive airway pressure therapy. Height, weight and waist circumference were measured in all patients. HbA1c values (defined as the proportion of hemoglobin that is glycosylated) were obtained from the patient's chart if assessed during the previous 3 months. The following tests were also collected: electrocardiogram, echocardiogram, renal function, cholesterol levels and thyroid function. Sleep complaints or symptoms of OSA were not used as selection criteria. During this period a total of 1520 patients were observed in the Diabetes Unit, of which 178 patients had type 1 DM. The patients were referred successively to the Sleep Unit if they met the criteria mentioned above. Three patients refused to undergo respiratory polygraphy. These were type 1 diabetic patients, not obese and without symptoms of OSAS.
Respiratory polygraphy (RP)The sleep study equipment measured body position, air flow via nasal cannula, oximetry, pulse rate, and respiratory effort via thoracic and abdominal bands (Embletta PDS 3.0, Flaga Medical, Iceland).
Total cessation of airflow for at least 10 s was defined as apnea (obstructive if respiratory efforts were present and central if respiratory efforts were absent). Hypopneas were defined as at least 30% reduction in thoraco-abdominal movement or airflow lasting at least 10 s which was associated with at least a 3% drop in oxygen saturation. The apnea–hypopnea index (AHI) was defined as the total number of obstructive apneas and obstructive hypopneas per hour of sleep. OSA severity categories were defined according to commonly used clinical cutoffs as follows: no OSA (AHI < 5); mild OSA (AHI ≥ 5 but <15); moderate OSA (AHI ≥ 15 but <30); and severe OSA (AHI ≥ 30). OSA was defined by AHI ≥ 5/h, excluding those with central apnea predominance, which was defined by a central apnea index/AHI ≥ 50%. All patients got more than 4 h of total sleep time during the RP.
Data analysisStatistical analysis was performed with SPSS for Windows 20.0 software package (SPSS, Inc; Chicago, IL). Mean and SD were used to express the central tendency and dispersion of continuous variables in normal distribution, and median and interquartile ranges are otherwise used. Comparisons between groups were performed with Student t tests or χ2 tests when appropriate. Pair-wise comparisons of continuous variables in patients with and without OSA were examined by t test and confirmed by the nonparametric Mann–Whitney test. A p-value ≤0.05 was considered to indicate statistical significance.
ResultsForty six patients were included (20 men and 26 women). The mean age was 51 ± 15 years and mean BMI was 29.0 ± 4.9 kg/m2. The mean Hba1c was 8.3 ± 1.2. Twenty three patients had type 2 DM and 23 patients had type 1 DM. Table 1 summarizes the demographic characteristics of the cohort. The sample included 10 lean, 16 overweight, and 20 obese patients. Snoring was the most frequently reported symptom; it was present in 20 patients (43.5%). Ten patients did not present any symptoms of OSAS. Regarding the major symptoms of OSAS, the presence of nocturnal apnea revealed a high specificity (100%) for the diagnosis of OSA, being present in only 6 patients with OSAS (20.7%). A total of 29 of the 46 patients (63%) had OSA (AHI > 5). Mild, moderate, and severe OSA was found in 32.6% (n = 15), 21.7% (n = 10), and 8.7% (n = 4) of the sample, respectively. Compared with patients without OSA, those with OSA were heavier and had a bigger neck circumference (Table 2). There were no differences in the Epworth sleepiness scale or in the duration of diabetes. There was no difference in the mean AHI and CT90 between obese and non obese patients (Table 3). The AHI was not correlated with the BMI (R2 = 0.08; p = 0.051). The predominant respiratory disturbances were obstructive apneas and hypopneas, rather than central apneas. Only one patient, with type 1 DM, had a central apnea index ≥5/h. With the exception of the mean age the two groups of diabetic patients were comparable (Table 4). The mean AHI was similar between type 1 and type 2 DM (15.7 ± 24.5 vs 11.6 ± 8.9; p = 0.455). The mean duration of diabetes was 17 ± 11 years and there was no relationship between this and the mean AHI (AHI 14.8 ± 20.8 with ≥10 years of duration vs. 9.4 ± 8.9 with less than 10 years; p = 0.39). Type 2 DM patients with poor glycemic control (Hba1c > 7.5%) had a higher mean AHI (14.3 ± 9.0 vs 6.4 ± 6.2; p = 0.038) (Graph 1). In ordinal regression analysis this difference did not remain significant after adjustment for BMI (p = 0.151).
Table 1. Demographic characteristics.
| Mean ± SD | |
| Age | 50.7 ± 15.1 |
| BMI, kg/m2 | 28.6 ± 4.9 |
| Waist circumference, cm | 97.8 ± 13.2 |
| Neck circumference, cm | 37.3 ± 10.2 |
| ESS | 7.7 ± 4.7 |
| Diabetes diagnosis, years | 17.3 ± 11.7 |
| HbA1c, % | 8.3 ± 1.2 |
Table 2. Differences between patients with and without OSA.
| Patients without OSA (n = 17) | Patients with OSA (n = 29) | p value | |
| Age | 45.2 ± 13.4 | 53.5 ± 15.4 | 0.07 |
| Male | 7 (41.2%) | 13 (44.8%) | 0.81 |
| BMI, kg/m2 | 26.7 ± 4.7 | 29.8 ± 4.1 | 0.02 |
| Waist circumference, cm | 95.3 ± 11.7 | 103.4 ± 13.7 | 0.06 |
| Neck circumference, cm | 37.4 ± 3.0 | 40.5 ± 5.1 | 0.03 |
| ESS | 7.5 ± 5.3 | 7.8 ± 4.3 | 0.81 |
| Diabetes diagnosis, years | 18.4 ± 10.4 | 16.3 ± 11.2 | 0.54 |
| HbA1C, % | 8.1 ± 1.3 | 8.4 ± 1.2 | 0.53 |
| Snoring | 7 (41.2%) | 13 (44.8%) | 0.81 |
| Witnessed apneas | 0 (0%) | 6 (20.7%) | 0.04 |
| Morning headaches | 8 (47.1%) | 9 (31.0%) | 0.28 |
| Restless sleep | 10 (58.8%) | 10 (34.5%) | 0.11 |
| Daytime hypersomnolence | 4 (23.5%) | 6 (20.7%) | 0.82 |
| Type 1 diabetes | 10 (58.8%) | 13 (44.8%) | 0.54 |
Table 3. Respiratory polygraphy data.
| Respiratory polygraphy | Non obese patients (n = 27) | Obese patients (n = 19) | p value |
| Total time of recording, min | 456.2 ± 66.1 | 408.7 ± 94.4 | 0.053 |
| AHI, events/h | 11.7 ± 12.6 | 16.4 ± 24.4 | 0.397 |
| CT90, % | 3.0 ± 5.3 | 8.7 ± 18.2 | 0.194 |
| Mean SpO2, % | 94.2 ± 1.7 | 93.0 ± 2.7 | 0.065 |
Table 4. Differences between type 1 and type 2 diabetes mellitus.
| DM type 1 (n = 23) | DM type 2 (n = 23) | p value | |
| Age | 38.7 ± 10.7 | 62.2 ± 7.7 | <0.001 |
| Male sex | 13 (56.5%) | 7 (30.4%) | 0.068 |
| BMI | 28.0 ± 5.1 | 29.2 ± 3.8 | 0.402 |
| HbA1c | 8.1 ± 0.6 | 8.4 ± 1.6 | 0.397 |
| Duration of diabetes | 17.3 ± 11.7 | 16.8 ± 10.2 | 0.881 |
| ESS | 9.0 ± 5.1 | 6.2 ± 3.8 | 0.060 |
| Mean AHI | 15.7 ± 24.5 | 11.6 ± 8.9 | 0.455 |
| CT90 | 4.7 ± 10.3 | 6.0 ± 14.6 | 0.551 |
| AHI > 5 | 13 (56.5%) | 16 (69.6%) | 0.542 |
| AHI > 15 | 6 (26.1%) | 8 (34.8%) | 0.749 |
Graph 1. Glycemic control in type 2 DM and apnea–hypopnea index.
DiscussionOur study indicates that OSA is highly prevalent in patients with type 2 and also in type 1 diabetes. There was no difference between type 1 and type 2 diabetes mellitus.
A total of 29 of our 46 subjects (63%) had OSA. These findings regarding the prevalence of OSA in patients with type 2 diabetes are consistent with the Sleep AHEAD (Action for Health in Diabetes) study.5 This study recruited 306 obese subjects with diabetes and found an OSA prevalence of 86% using full overnight polysomnography (PSG), where BMI and waist circumference were identified as significant predictors of OSA. That study cohort was composed of obese subjects (mean BMI, 36.5 ± 5.8 kg/m2), which could have contributed to the high prevalence. Similar high prevalence of OSA, up to 77%, was reported in another clinic-based study including 60 subjects with diabetes, with mean BMI of 33.8 kg/m2.13 In our study the prevalence of OSA was lower than that study which could be explained by the lower BMI of our cohort and we also used respiratory polygraphy to identify patients with OSA. Mild, moderate, and severe OSA was found in 32.6% (n = 15), 21.7% (n = 10), and 8.7% (n = 4) of the sample, respectively. We observed lower rates than the sleep AHEAD study. Our study population included patients with type 1 and type 2 diabetes mellitus, with a slight predominance of female sex, the majority were non obese patients and 21.7% patients had a BMI < 25 kg/m2. The high percentage (69.6%) of female patients with type 2 DM may explain the lower prevalence of OSAS. OSAS has been considered predominantly a disease of middle-aged obese men and it is generally accepted that this pathology is twice as common in men than in women.14 The female patients usually have a higher percentage of mild OSAS and a higher BMI compared to male patients.15 The Sleep Heart Health Study involving older individuals (about 50% more than 65 year of age), for which the diagnosis of diabetes was based on self-report only, found an OSA prevalence of 58%,7 similar to our study.
Our study also demonstrated a high prevalence of OSA in patients with type 1 DM. Borel et al.12 observed a prevalence of 40% in 37 non-obese adult patients with type 1 diabetes mellitus. Our study differed in that obese patients with type 1 DM were also included and we found a slightly higher prevalence of OSA (56.5%).
There were no differences between patients with type 1 and type 2 DM, in this way the patients with type 1 DM present a similar IAH at a much younger age in relation to the patients with type 2 DM. This difference may indicate the early development of OSA in patients with type 1 DM, constituting a more severe form of the disease. Our study cannot determine the mechanisms responsible for this situation. Obstructive sleep apnea is caused by pharyngeal occlusion due to alterations in upper airway mechanical properties and/or disturbances in neuromuscular control.16 The presence of pharyngeal neuropathy may cause the development of OSA in patients with type 1 DM. Previous studies on diabetic patients showed that autonomic neuropathy is associated with a relatively high frequency of OSA.17 The underlying mechanism remains unknown but could be related to an impairment of the upper airway reflexes, possibly due to alterations of the autonomic nervous fibers involved in their regulation.17
We did not use the gold standard test for diagnosis of sleep apnea. Dingli et al.18 reported that simplified polysomnography is more likely to yield underestimations than overnight sleep polysomnography. Our results cannot be applied to the diabetic population in general; the study included only diabetes patients followed as outpatients in hospital care. Despite the fact that the symptoms of OSA did not constitute a selection criterion we cannot exclude the existence of selection bias, due to the recruitment method. On the other hand we studied only a small fraction of the diabetic population followed in the Diabetes Unity, which may not have been representative. Patients with prior diagnosis of sleep apnea were not included in this analysis and therefore we cannot determine a real prevalence of sleep apnea in diabetes patients. However the high percentage of patients with sleep apnea that remained undiagnosed is interesting.
There are only a few published studies that have examined the association between the severity of OSA and the HbA1c levels. HbA1c is considered the major index for monitoring glycemic control in diabetic patients. Glycemic control in patients with DM is well known to be subjected to various DM-related factors, including antidiabetic medications, duration of DM, physical activity, and diet, which could interfere with any effect that OSA may pose. HbA1c does not reflect glucose fluctuations and might not be the major clinical indicator of glycemic control in the cases where frequent hypoglycemic and/or hyperglycemic episodes are present.19 Monnier et al.20 demonstrated that glucose variability is a risk factor for complications independent of HbA1c in type 2 DM. To examine the impact of untreated OSA on glucose control in type 2 diabetes, Aronsohn et al.13 measured hemoglobin A1c in 60 consecutive patients with diabetes. OSA (AHI > 5) was present in 77% of patients with type 2 diabetes and 38% of the patients had moderate or severe OSA (AHI > 15). Compared with patients without OSA, the adjusted mean hemoglobin A1C was increased by 1.49% in patients with mild OSA, 1.93% in moderate OSA, and 3.69% in severe OSA. OSA severity was associated with a poorer glucose control, independently of obesity and other confounding factors. These findings were corroborated by a recent large multinational study, although in the analysis of the 6.117 participants of the European Sleep Apnea Cohort (ESADA) the adjusted mean HbA1c levels were only 0.72% higher in severe OSA patients when compared with patients without OSA.21
We also found that type 2 DM patients with a poor metabolic control had a significant higher apnea–hypopnea index. However, this difference did not remain significant after correction for BMI, contrary to previous studies.6, 7, 8, 9 It has been postulated that abdominal adiposity affects the degree of glycemic control in diabetic patients.22 On the other hand, it is possible that other confounding factors interfered with HbA1c and did not let OSA to have an effect. West et al.23 also did not find a correlation between OSA severity and HbA1c in the subgroup of primary care patients.
In type 1 diabetes it is possible that disturbed sleep characteristics influence glucose metabolism. However the effects of impaired sleep characteristics may not simply be reflected in HbA1c values because intensive glucose control and frequent, appropriate adjustments of insulin doses in patients at risk might have obtunded the effects of sleep characteristics on glucoregulation.11
Ronksley et al.24 recently published data on the prevalence of DM in 1717 patients with OSA and 432 without and found that DM is associated with severe OSA (respiratory disturbance index > 30/h) even after adjustment for age, body mass index, sex, neck circumference, and smoking status. More recently, the analysis of the 6.117 participants on ESADA study showed that the prevalence of type 2 DM increased with OSA severity. The patients with OSA had an increased risk of type 2 DM, independently of obesity and other confounding variables.22
Due to the small size of our sample, our study is mainly descriptive not allowing us to achieve any statistically significant results. Despite the relatively low prevalence of obesity in our sample, this study did not identify a possible role of diabetes independently of obesity.
Our study reveals that the majority of patients with type 2 diabetes have undiagnosed OSA, and that untreated OSA could be associated with poorer glucose control. The authors stress the high prevalence of OSA in younger patients with type 1 DM. Further studies are required in patients with type 1 DM to confirm these observations and for a better understanding of the underlying mechanisms and clinical implications of the relationship between OSAS and type 1 DM.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Received 10 October 2013
Accepted 12 July 2014
Corresponding author. jorge_mvale@hotmail.com