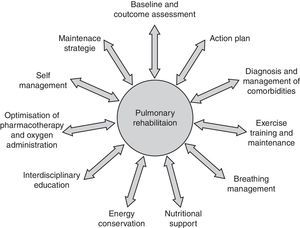
Built around exercise training, pulmonary rehabilitation (PR) is a multidisciplinary, evidence‐based, comprehensive approach to working with the patient as a whole and not just the pulmonary component of the disease. Integrated into the individualized treatment, this intervention aims to reduce symptoms, optimize functional status, increase participation in daily life, and reduce health care costs through stabilizing or reversing systemic manifestations of the disease. Although there are many other components that should be considered to manage the impairment and symptom burden, supervised exercise training is considered the cornerstone of effective pulmonary rehabilitation. This paper addresses our clinical experience at Institut universitaire de cardiologie et de pneumologie de Québec to assess and manage exercise training in line with the current recommendations and guidelines surrounding PR.
Construída com base no exercício físico, a reabilitação pulmonar (RP) é uma abordagem multidisciplinar, fundamentada e abrangente para trabalhar com o doente como um todo, e não apenas com a componente pulmonar da doença. Integrado no tratamento individual, esta intervenção visa reduzir os sintomas, optimizar o estado funcional, aumentar a participação na vida diária e reduzir os custos do tratamento de saúde, através da estabilização ou inversão das manifestações sistémicas da doença. Embora existam muitos outros componentes que devem ser tidos em consideração para gerir o peso da incapacidade e dos sintomas, o exercício físico supervisionado é considerado o fundamento da reabilitação pulmonar eficiente. Este documento trata da nossa experiência clínica no Institut universitaire de cardiologie et de pneumologie de Québec para avaliar e gerir o exercício físico em linha com as recomendações e orientações actuais envolvendo a RP.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality wordwide and it is expected that by 2020 COPD will be the fifth most burdensome disease and the third leading cause of mortality.1 The natural course of the disease is punctuated by episodes of acute exacerbation which contribute to the increased morbidity and mortality and socioeconomic burden associated with COPD.2 Patients with COPD frequently show exercise intolerance and dyspnea, reduced ability to participate in activities of daily living, reduced health‐related quality of life and increased use of health care resources.3 If optimal bronchodilation can be seen as a first step in the treatment of patients with COPD, more effective treatments (e.g., improvements in exercise performance, symptoms, and health‐related quality of life) are often achieved after pulmonary rehabilitation (PR).4 Comprehensive PR aims at tackling the systemic consequences of COPD and also the behavioral and educational shortcomings observed in many patients and it is now widely recognized as an effective and key intervention in the management of COPD.1,5–10 It is also suggested that PR appears to be cost‐effective, it decreases utilization of health care services.11,12
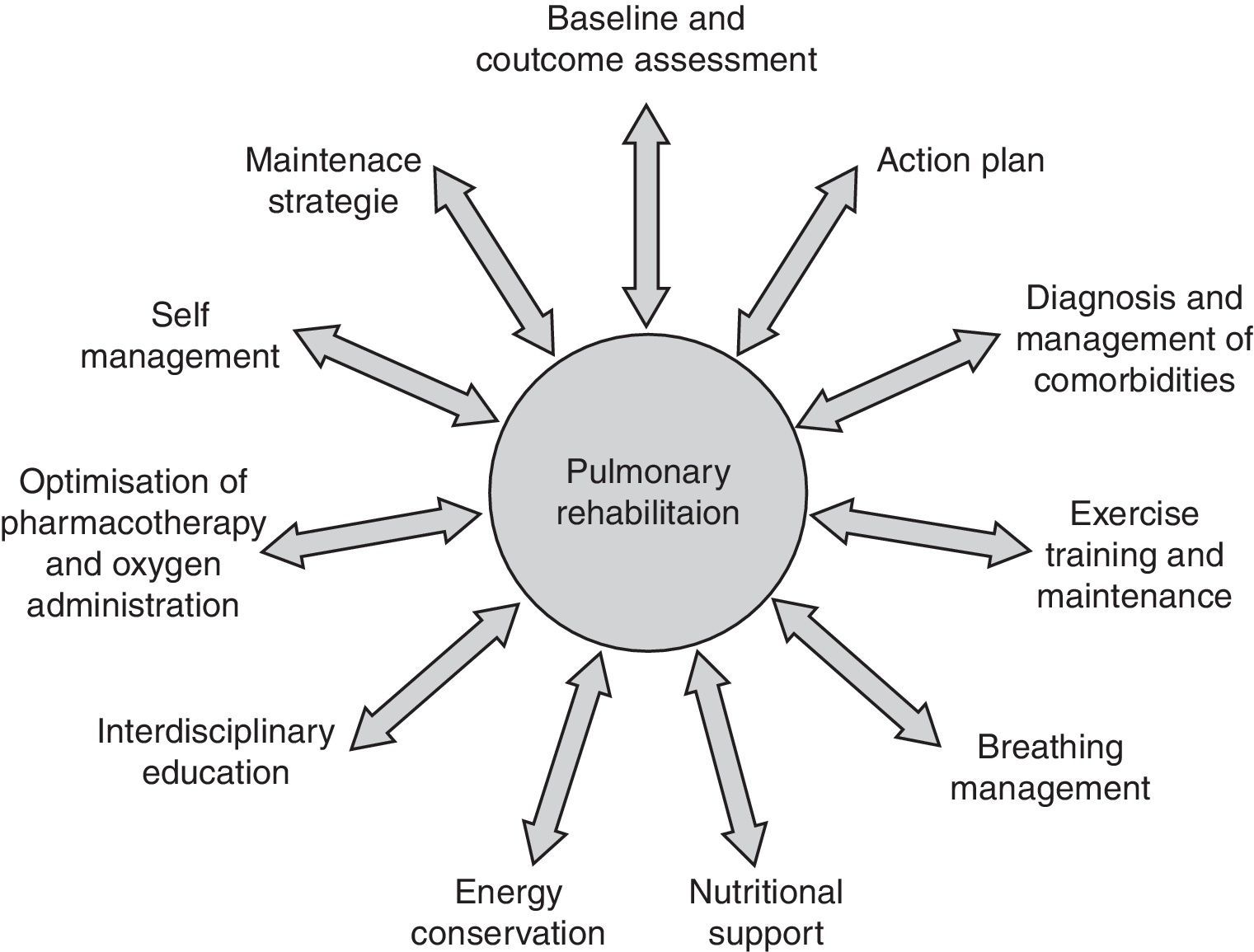
Pulmonary rehabilitation definition, concept and settingBuilt around exercise training, PR is a multidisciplinary, evidence‐based comprehensive approach to addressing the patient as a whole and not only the pulmonary component of the disease (Fig. 1). Integrated into the individualized treatment of the patient, PR is individually tailored and designed to reduce symptoms, optimize functional status, increase participation, and reduce health care costs through stabilizing or reversing systemic manifestations of the disease.5,8,10,13–17
In a broader sense, PR includes a spectrum of strategies integrated into the lifelong management of patients with chronic respiratory disease and involves a dynamic and active collaboration between patients, families, and health care providers. Pulmonary rehabilitation typically includes individualized patient assessment, supervised exercise training, self‐management education, psychosocial support and nutritional counseling.5,10 Patients are usually enrolled in a 6–12‐week exercise program which they attend 2–3 times per week.
Individualized patient assessment should include medical history, physical exam and accurate diagnosis of their respiratory condition based on pulmonary function testing and detection of comorbidities that may interfere with the rehabilitation process.10 Baseline assessment should also comprise measures of exercise capacity, symptoms and quality of life.18 Hypoxemia at rest and during effort is what should be looked for.
Exercise training is the cornerstone of effective PR and preferably includes both aerobic and muscle training. To achieve clinically relevant results, training should be closely supervised and performed for 30–45min, at least 3 days per week. Patients should undertake a minimum of 20 sessions, but longer programs produce broader and more long‐lasting results.5,16,19–22
Nutritional intervention should be considered for patients with body composition abnormalities such as cachexia and obesity which is becoming one of the most prevalent nutritional issues in COPD.23 Patients with fears and anxiety may benefit from psychosocial support and the integration of occupational therapy in PR can improve autonomy in activities of daily living. Moreover, in order to facilitate chronic disease self‐management there are other areas of importance: these include approaches designed to (1) facilitate smoking cessation; (2) optimize pharmacotherapy; (3) assist with early identification and treatment of acute exacerbations; (4) manage acute dyspnea; (5) increase physical activity; (6) improve body composition; (7) promote mental health; (8) facilitate advance care planning; and (9) establish social support networks.24 Finally, strategies to promote a more active lifestyle following PR, such as the implementation of a home exercise program should be considered.
Pulmonary rehabilitation can be effective as inpatient, hospital‐ or community‐based outpatient, or home‐based programs.5,15,25 Inpatient programs are generally more expensive and suitable for patients with limited transportation or severe deconditioning requiring specific resources such as nutritional supplementation or training for home ventilation.8 Because outpatient programs offer rehabilitation with secure and predictable improvements at a relatively low cost, multidisciplinary PR programs are typically implemented in outpatient hospital‐ or community‐based settings. Home‐based exercise programs are also effective in improving exercise tolerance and quality of life.26,27 Home‐based rehabilitation is well suited for highly motivated, self‐directed individuals but appears to be less successful with severe, homebound patients.28,29 Home‐based rehabilitation is particularly effective in maintaining improvements obtained in an outpatient setting30
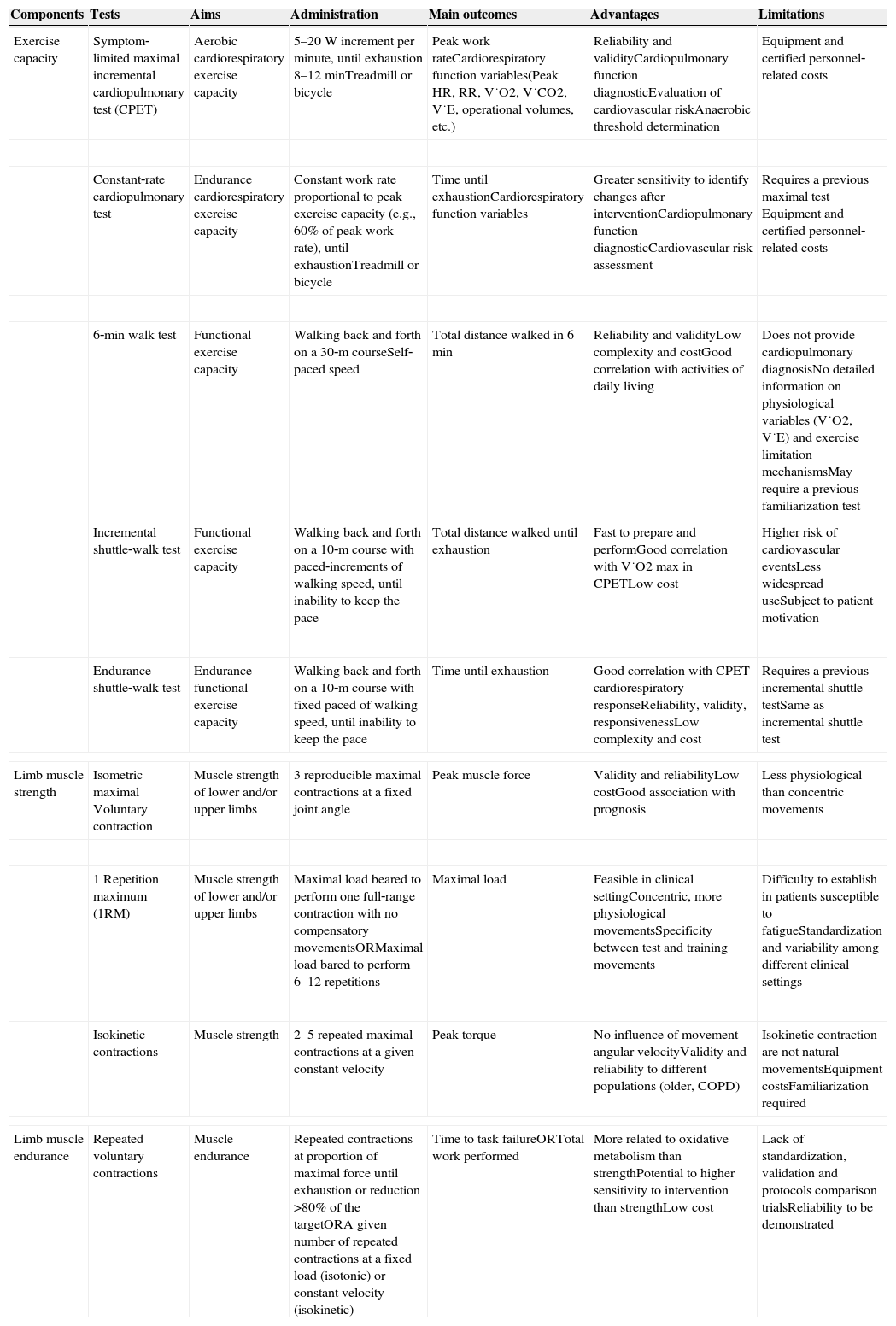
Assessment in pulmonary rehabilitationIn order to guide assessment and prescription of exercise training in PR programs, cardiorespiratory exercise capacity and limb muscle function are the essential components to be evaluated. As there are several tests available; a summary of the most common validated tests, as well as their essential characteristics, is presented in Table 1. In clinical practice, the training objectives and the availability of resources will influence the choice of the tests to be used.
Physical function assessment examples.
| Components | Tests | Aims | Administration | Main outcomes | Advantages | Limitations |
|---|---|---|---|---|---|---|
| Exercise capacity | Symptom‐limited maximal incremental cardiopulmonary test (CPET) | Aerobic cardiorespiratory exercise capacity | 5–20W increment per minute, until exhaustion 8–12minTreadmill or bicycle | Peak work rateCardiorespiratory function variables(Peak HR, RR, V˙O2, V˙CO2, V˙E, operational volumes, etc.) | Reliability and validityCardiopulmonary function diagnosticEvaluation of cardiovascular riskAnaerobic threshold determination | Equipment and certified personnel‐related costs |
| Constant‐rate cardiopulmonary test | Endurance cardiorespiratory exercise capacity | Constant work rate proportional to peak exercise capacity (e.g., 60% of peak work rate), until exhaustionTreadmill or bicycle | Time until exhaustionCardiorespiratory function variables | Greater sensitivity to identify changes after interventionCardiopulmonary function diagnosticCardiovascular risk assessment | Requires a previous maximal test Equipment and certified personnel‐related costs | |
| 6‐min walk test | Functional exercise capacity | Walking back and forth on a 30‐m courseSelf‐paced speed | Total distance walked in 6min | Reliability and validityLow complexity and costGood correlation with activities of daily living | Does not provide cardiopulmonary diagnosisNo detailed information on physiological variables (V˙O2, V˙E) and exercise limitation mechanismsMay require a previous familiarization test | |
| Incremental shuttle‐walk test | Functional exercise capacity | Walking back and forth on a 10‐m course with paced‐increments of walking speed, until inability to keep the pace | Total distance walked until exhaustion | Fast to prepare and performGood correlation with V˙O2 max in CPETLow cost | Higher risk of cardiovascular eventsLess widespread useSubject to patient motivation | |
| Endurance shuttle‐walk test | Endurance functional exercise capacity | Walking back and forth on a 10‐m course with fixed paced of walking speed, until inability to keep the pace | Time until exhaustion | Good correlation with CPET cardiorespiratory responseReliability, validity, responsivenessLow complexity and cost | Requires a previous incremental shuttle testSame as incremental shuttle test | |
| Limb muscle strength | Isometric maximal Voluntary contraction | Muscle strength of lower and/or upper limbs | 3 reproducible maximal contractions at a fixed joint angle | Peak muscle force | Validity and reliabilityLow costGood association with prognosis | Less physiological than concentric movements |
| 1 Repetition maximum (1RM) | Muscle strength of lower and/or upper limbs | Maximal load beared to perform one full‐range contraction with no compensatory movementsORMaximal load bared to perform 6–12 repetitions | Maximal load | Feasible in clinical settingConcentric, more physiological movementsSpecificity between test and training movements | Difficulty to establish in patients susceptible to fatigueStandardization and variability among different clinical settings | |
| Isokinetic contractions | Muscle strength | 2–5 repeated maximal contractions at a given constant velocity | Peak torque | No influence of movement angular velocityValidity and reliability to different populations (older, COPD) | Isokinetic contraction are not natural movementsEquipment costsFamiliarization required | |
| Limb muscle endurance | Repeated voluntary contractions | Muscle endurance | Repeated contractions at proportion of maximal force until exhaustion or reduction >80% of the targetORA given number of repeated contractions at a fixed load (isotonic) or constant velocity (isokinetic) | Time to task failureORTotal work performed | More related to oxidative metabolism than strengthPotential to higher sensitivity to intervention than strengthLow cost | Lack of standardization, validation and protocols comparison trialsReliability to be demonstrated |
Cardiopulmonary exercise testing is the best recognized way to assess exercise capacity and cardiovascular risk. The ATS‐ACCP statement provides a good description of the recommendations for cardiopulmonary exercise tests.31
Incremental maximal cycling or treadmill cardiopulmonary tests allow medical staff to prescribe precisely the intensity endurance training based on physiological responses to variables such peak heart rate, ventilation (V˙E), oxygen consumption (V˙O2), CO2 output (V˙CO2) and respiratory exchange ratio (RER). A constant work rate cardiopulmonary test in which patients are asked to exercise as long as possible at a submaximal work rate is also recommended. The constant work rate test is clinically pertinent to PR programs because of its excellent sensitivity which enables it to identify changes after exercise training or other therapeutic interventions.32,33
Although cardiopulmonary exercise testing is relevant and recommended, it is also more expensive, complex and requires more resources and so tends to be implemented more frequently in highly specialized centers. In addition to the laboratory based tests of exercise capacity, field‐based tests can be undertaken with minimal resources. Although less complex and less costly, they must not be simply considered as substitutes for cardiopulmonary tests.
Walking testsThe 6‐min walk‐test (6MWT) is the most widely used field‐based test in clinical and research settings.34 Walking is a reflection of the integrated respiratory, cardiovascular and neuro‐muscular responses to exercise. The distance walked results from the combination of all systems involved in the task of walking and it would be difficult to know which of these systems plays a major limiting role in task failure. Patients who experience severe breathlessness, for example, might reduce walking speeds during self‐paced tests to avoid unpleasant respiratory symptoms. This test is also believed to reflect functional status in daily activities better than the cardiopulmonary tests.34,35 In general, 6MWT is considered a submaximal functional test. Due to the familiarization effect, performing at least two tests is recommended.34 Using the established recommended protocol is critical to obtaining reliable, valid, and reproducible test results.34
Walking test may also be carried out using incremental and endurance shuttle walking tests. The incremental shuttle walking test is an externally paced maximal exercise test; the speed of walking is controlled by a series of pre‐recorded signals played from a CD. The speed of walking increases until the participant can no longer continue. For the endurance shuttle walking test patients need to walk as long as possible at a predetermined speed calculated from the incremental shuttle walking test. Detailed indications, contraindications and technical aspects are available elsewhere.34,36
Finally, the assessment of symptom perception during both laboratory and field‐based tests is strongly recommended and is a useful guide for exercise prescription.31 Thus, patients may easily indicate their perceptions of dyspnea and leg fatigue throughout the tests by the 10‐point modified Borg scale, which is the most widely used in PR programs.
Assessment of limb muscles functionWe encourage routine assessment of limb muscle function before pulmonary rehabilitation as this provides important information about the mechanisms of exercise limitation. Also, knowledge of limb muscle function may help in the drawing up of individual training programs.
Muscle strengthThere are several tests available to quantify muscle strength.37 Isometric maximal voluntary contractions using strain gauge remains a preferred method of easily assessing volitional strength in clinical practice.38 Muscle contractions are performed at a fixed joint angle. It is a highly reproducible and sensitive method when standardization is respected. However, it can be less relevant to movements made during training sessions which are often isotonic concentric contractions, such as weight lifting. For example, a one‐repetition maximum (1RM) is a widely used evaluation method in which strength is defined as “the maximal amount of weight that can be lifted through the full range of motion, for one repetition, with proper form”.39 However, choosing this optimal load may be challenging and time‐consuming for patients who are more susceptible to muscle fatigue, such as COPD patients.40 An example of a 1RM test is provided in Table 1. The advantage is that this load may serve as a baseline from which to start training.
Many other tools and equipment may be useful for measuring muscle strength such as manual muscle testing, isokinetic dynamometers, hand‐held dynamometers, hydraulic resistance, peripheral and transcranial magnetic stimulation. Careful analysis of each assessment tool validity, reliability and cost‐benefit is mandatory before implementing their use.
Muscle enduranceThe capacity of muscles to sustain a required task for as long as possible, labeled as “muscle endurance”, is more often associated with muscle aerobic capacity41 and less determined by muscle mass than muscle strength42. Moreover, measures of muscle endurance may be more sensitive to detecting changes associated with exercise training than muscle strength. In addition, muscle endurance cannot be predicted from muscle strength, providing an additional rationale for its measurement before exercise training.43 Unfortunately, we still do not know which assessment tools would best reflect improvements in muscle endurance, especially considering the nature and intensity of the exercise training protocol applied. In addition, no direct comparisons between different endurance protocols, no standardization and no reference values are available. These deficiencies may explain why assessment of limb muscle endurance is not more widely applied in clinical practice. In Table 1, some suggestions are provided given the current available data on muscle endurance assessment. Consideration should be given to the fact that exercises promoting muscle endurance may help patients to develop higher muscle tolerance, thereby favoring tolerance during whole‐body exercise training such as walking and cycling.
Quebec experienceThe current format of the PR program at the Institut universitaire de cardiologie et de pneumologie de Québec started in 1992 under the leadership of Dr. Roger Belleau, a visionary pulmonologist, who devoted his medical practice to the improvement of care for patients with chronic respiratory disease. The development of a PR program was not an obvious thing to do back in early 90s when skepticism about the effectiveness of this intervention was common in the medical community.44 Since then, PR has come a long way and is now considered as a standard of care in COPD with all the benefits supported by an important body of literature already reported in the first part of this article.10,17
Our PR program is a multidisciplinary 12‐week outpatient, hospital‐based program. It includes pre‐program assessment, self‐management teaching program, exercise training program, maintenance program and post‐program assessment. We are currently implementing a well‐structured home‐rehabilitation program to be carried out in the community but coordinated from the hospital.
The patients are usually referred by the doctors or the nurses and respiratory therapists of the COPD clinic which offers a unique opportunity of convincing patients about the efficacy of pulmonary rehabilitation. The coordinating nurse takes patients through the evaluation process and self‐management program. Exercise specialists (kinesiologists and physiotherapists) supervise the exercise component of the program and offer advice on the selection of the most appropriate strategies for maintenance strategy and for the development of a more active daily life.
Pre‐program assessmentAll the patients undergo a maximal incremental and symptom‐limited exercise test on a cycle ergometer.45 Maximal exercise testing provides peak work rate, peak heart rate, and also information on arterial blood pressure, pulse oxygen saturation (SpO2), dyspnea, leg fatigue and ECG changes during effort. This evaluation in the context of supervised PR allows for the planning of the intensity and progression of aerobic training. An endurance test is also routinely performed on a cycle ergometer at a constant work load equivalent to 80% of peak work load during incremental testing. We measure the time that can be sustained at a pedaling rate above 40rpm. Six‐min walk distance, spirometry, body composition analysis by bioelectrical impedance, waist circumference and arterial blood gases are also obtained. In patients with COPD, the impact of disease on a person's life is determined by the COPD Assessment test. (CAT) questionnaire.46 Possible barriers that could impinge on the progression and success of the program are identified in every patient. Finally, quantification of physical activities during daily life, respiratory muscle strength and 1 maximal repetition (1RM) for targeted strength training exercises are also done for clinical research purposes.
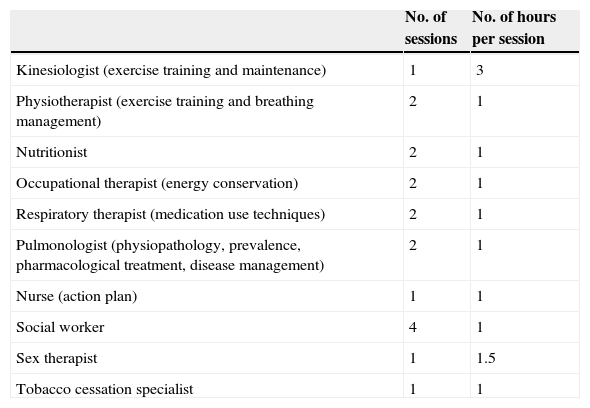
Self‐management teaching programPatients are invited to attend group teaching sessions with their significant others on a variety of themes, with the common goal of promoting self‐management strategies based on the model “Living well with COPD” (link: http://www.livingwellwithcopd.com/; password: copd). Overall, 18 sessions are be offered (Table 2).
Self‐management teaching program at IUCPQ.
| No. of sessions | No. of hours per session | |
|---|---|---|
| Kinesiologist (exercise training and maintenance) | 1 | 3 |
| Physiotherapist (exercise training and breathing management) | 2 | 1 |
| Nutritionist | 2 | 1 |
| Occupational therapist (energy conservation) | 2 | 1 |
| Respiratory therapist (medication use techniques) | 2 | 1 |
| Pulmonologist (physiopathology, prevalence, pharmacological treatment, disease management) | 2 | 1 |
| Nurse (action plan) | 1 | 1 |
| Social worker | 4 | 1 |
| Sex therapist | 1 | 1.5 |
| Tobacco cessation specialist | 1 | 1 |
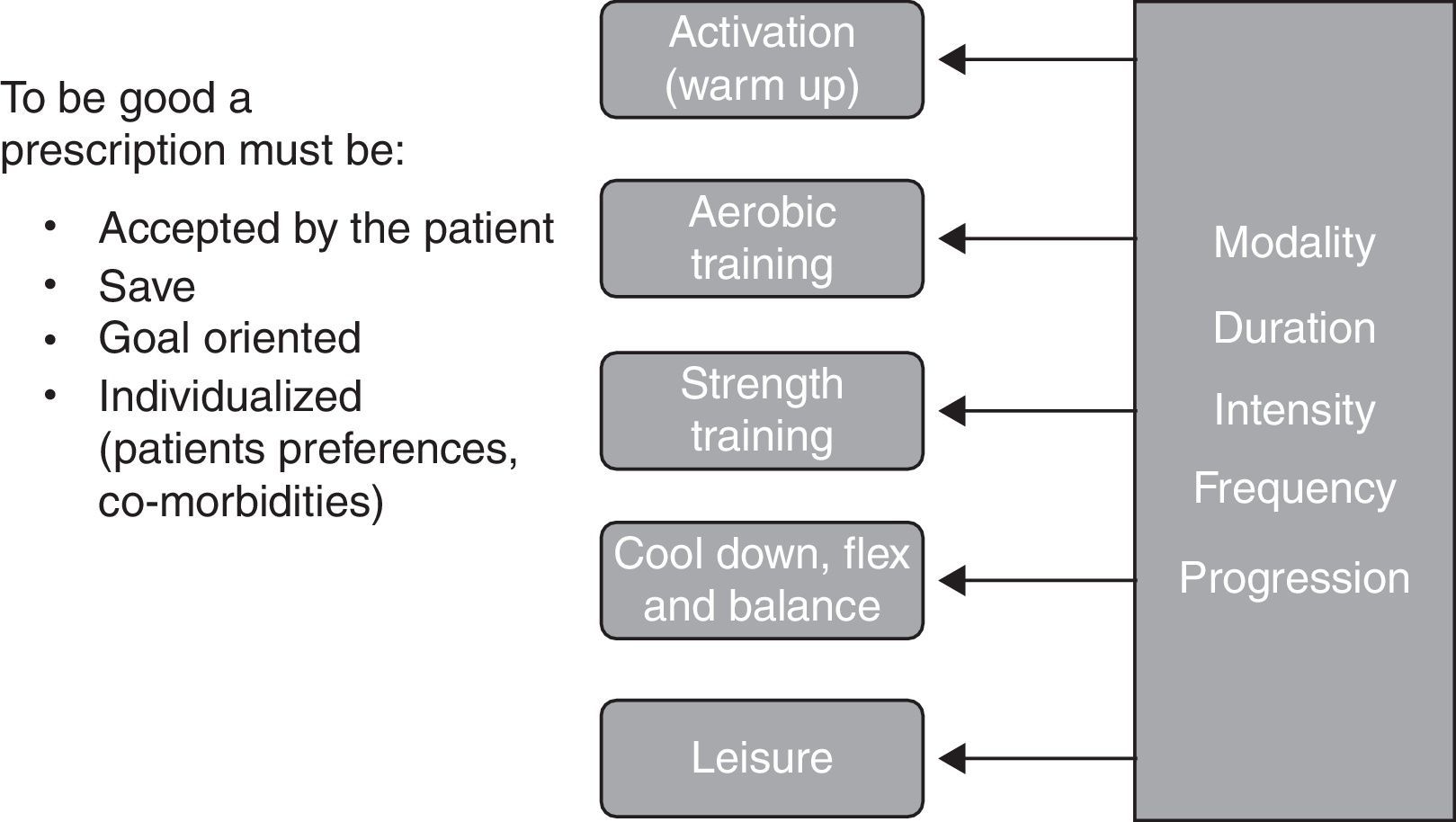
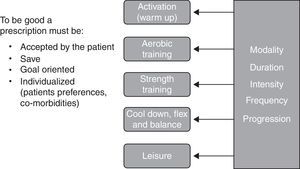
Prescribing exercise requires knowledge and experience to interpret and use elements of the assessment, to plan a safe, challenging and tailored exercise program with clear objectives and an appropriate progression to optimize benefits. Exercise prescription takes into account respiratory and muscle limitations as well the indication and contraindications for exercise, the medication, the comorbidities and the particular preferences of each patient. As shown in Fig. 2, an exercise prescription should be composed of different parts, each divided into modality‐duration‐intensity‐frequency‐progression.
In our program the activation (warm up) is the initial part of the aerobic training and it is done with the same modality for a few minutes, up to 5min, at a very low intensity (20–40% of the heart rate (HR) reserve). It is followed by aerobic training which is usually a combination of cycling and walking. During the first weeks of the program, short bouts of exercise are favored but the objective is to train 3 times per week, for 30–40min doing interval or continuous training at a heart rate that may reach the heart rate recorded at 80% of peak incremental exercise testing or 80% of the HR reserve. To help exercise intensity progression, we work on respiratory control which is helpful in reducing dyspnea perception.
Muscle training is usually conducted after aerobic training, when the body is well warmed up. Our muscle training program is aimed at promoting muscle strength and hypertrophic gains. In general, large muscle chains are chosen and a selection of exercises is prescribed 3 times per week. Each exercise should be performed for 2–3 series of 10 repetitions maximum. The use of training devices with selective weights is the usual modality but also available are isometric exercises, free weights, elastics bands, and medicine balls. To assure good progression, the number of repetitions should always be increased before increasing the weight.
The exercise session is completed with relaxation, cool down, flexibility and balance exercises that are tailored to the patients’ needs.
Once a week, we have a period dedicated to recreational activities. We choose different ludic activities or sports to build team spirit and simply have fun. Leisure activities should be discussed and promoted outside of the program.
Supervision of the programExercise sessions are directly supervised by 2 exercise specialists, one kinesiologist and one physiotherapist, combining their expertise. Pursed lips breathing and breathing control are reinforced throughout the program. The use of the BORG scale is also promoted and is a key tool for assessing the perception of effort, fatigue and dyspnea. The professionals are always attentive and responsive to signs and symptoms related to effort intolerance, blood pressure, heart rate, glycemia (in diabetic patients), edema among others.6,10,47
The exercise specialists are experienced and, with a combination of knowhow and creativity, adapt the program to every patient's goals, capacities and limitations.
Maintenance programDifferent maintenance strategies are offered to the patients during the program. It is important to prepare for the end of the program in such a way that they do not feel abandoned or without options; in this type of situation, they would be likely to return to sedentary lifestyle after rehabilitation. Home exercises are also taught during the self‐management sessions.
Post‐program assessmentEvaluating the patients at the end of the program will provide valuable information about the efficacy of the intervention and should help motivate patients to pursue an active life when they see the progress they have made. Demonstrating efficacy has also been instrumental in obtaining institutional recognition and financial support. The post‐program evaluation includes constant cycling workrate exercise performed at pre‐program intensity, 6‐min walk test, body composition assessment (bioelectrical impedance and waist circumference) and the COPD Assessment Test (CAT).
Home‐rehabilitation programUndoubtedly, translating gains in exercise capacity with PR into a more active lifestyle is one of the main challenges in rehabilitation.16,19,24,48 Because home‐based rehabilitation may be the most convenient, it may appear to be superior to center‐based programs in terms of long term adherence to exercise.49 Thus, patients are encouraged to continue their training by either remaining in a hospital‐ or community‐based program, or by initiating a home‐based program. We are actively promoting home‐based PR and hopefully will implement it locally on a bigger scale (for example, by encouraging the implementation and participation in different research projects such as home based PR or telerehabilitation25,50,51).
DiscussionReferral to a pulmonary program is an integral part of COPD management. Patient evaluation is mandatory before and after PR and ideally during any long‐term follow‐up assessment to quantify the impact of PR and to guide management. In addition to valid, responsive and interpretable laboratory‐based exercise tests, field‐based tests may be useful because of their ease of administration and relevance to daily life. Remaining challenges are the limited access to PR programs52 as well as maintaining initial benefits and their translation into active lifestyles.48 In this paper we have shared our own views about PR but we clearly encourage the creation of a diversity of programs and intervention to promote an active life style in a larger COPD population. These programs, however small they might be, should be well structured, done by a multidisciplinary team specifically trained in PR and tailored to the patients’ needs. Moreover, when a program is available, its success clearly relies on the quality of care but also on the amount of promotion that we do. The local medical staff need to be aware that they can refer patients to a program but the information should transcend the local institution to ensure the sustainability of the program.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.