Poorly reversible airflow obstruction may or may not be related to smoking.
ObjectivesTo describe patients with severe obstructive lung disease including etiology, imaging, functional aspects, systemic manifestations, and the pattern of bronchodilator response.
MethodsSixty-eight patients (age 55.9±13.7 years, FEV1 [forced expiratory volume in one second] 31.9±10.2% predicted) underwent spirometry, evaluation of body mass composition, 6-minute walk test, X-ray, thorax high-resolution CT scanning, and clinical evaluation.
ResultsOf 68 patients enrolled, 37 had chronic obstructive pulmonary disease (COPD) and 31, extensive bronchiectasis. Among COPD patients the CT scans showed emphysema in 78.4%, and bronchiectasis in 48.6%. There were no significant differences between smokers and non-smokers, except for vital capacity, significantly smaller in non-smokers (p<0.001). We found 29 and 20 volume responders (VR) according to Paré et al. (FEV1/FVC>1=flow responder or <1=VR) and ATS/ERS criteria, respectively. According to Paré et al. criteria, there were 18 patients with FEV1<30% predicted among 29 VR, and 12 with FEV1<30% predicted among 39 without volume response (p=0.0101).
ConclusionsIn patients with severe obstruction, smoking does not appear to be relevant in determining functional or systemic differences, and Paré et al. criteria can detect more VR. Bronchiectasis is a common finding in severe COPD.
A obstrução das vias respiratórias pouco reversível pode ou não estar relacionada com o tabagismo.
ObjetivosDescrever pacientes com doença pulmonar obstrutiva grave, incluindo etiologia, aspectos dos exames de imagem, parâmetros funcionais, manifestações sistémicas, e o padrão da resposta ao broncodilatador.
MétodosSessenta e oito pacientes (idades de 55,9±13,7 anos, FEV1 [volume expiratório forçado num segundo] 31,9±10,2% previsto) foram submetidos a espirometria, avaliação da composição de massa corporal, teste de caminhada de 6 minutos, radiografia, tomografia computorizadas (TAC) de alta resolução do tórax, e avaliação clínica.
ResultadosDos 68 pacientes inscritos, 37 sofriam de doença pulmonar obstrutiva crónica (DPOC) e 31 de bronquiectasias extensa. Entre os pacientes com DPOC, as tomografias computadorizadas apresentaram enfisema em 78,4% e bronquietasias em 48,6%. Não existiram diferenças significativas entre os fumadores e os não-fumadores, exceto para a capacidade vital, significativamente inferior nos não-fumadores (p < 0,001). Encontramos 29 respondedores de volume (RV) pelos criterios de Paré et al. (VEF1/CVF > 1= respondedor de fluxo, se > 1 respondedor de volume), e 20 RV pelos criterios da ATS/ERS. De acordo com os critérios de Paré et al., existiam 18 pacientes com FEV1< 30% previsto entre os 29 RV, e 12 com FEV1 < 30% previsto entre os 39 sem resposta a uma prova de volume (p = 0,0101).
ConclusõesEm pacientes com obstrução grave, o tabagismo não parece ser relevante na determinação de diferenças funcionais ou sistémicas, e os critérios de Paré et al. podem detetar mais RV. A bronquiectasias é uma descoberta comum em DPOC grave.
It is quite clear nowadays that the most frequent cause of chronic and progressive airway disease that leads to chronic respiratory failure is Chronic Obstructive Pulmonary Disease (COPD) related to tobacco use. COPD is diagnosed if poorly reversible airflow obstruction can be demonstrated by spirometry and the exposure to noxious gases is present.
Poorly reversible airflow obstruction is not confined to smokers. Kohansal et al.,1 in a study about the natural history of chronic airflow obstruction, analyzed the Framingham Offspring cohort, who were followed for 23 years. One third of the individuals, who continued to smoke, developed airflow obstruction during follow-up. But so did 7.4% of never-smoker males and 5.6% of never-smoker females.
The classic work of Fletcher et al.2 on the natural history of COPD did not include non-smokers and therefore provides no information about the subjects who do not smoke but do have chronic airflow obstruction. In the United States, 20% of the patients who have obstruction in spirometry and 20% of those who die of COPD are non-smokers.3–6 Few studies have been performed in this group and little is known about its natural history and clinical course.
Mucus hypersecretion, one of the classic features of COPD, is associated with exacerbations and both situations are related to progressive loss of airflow.7,8
On the other hand, patients with hypersecretion unrelated to COPD may present functional and clinical pictures that overlap with chronic bronchitis associated with smoking.9
In addition to the symptoms and the spirometric changes, other similarities may exist between COPD and bronchiectasis from various causes: the predominant neutrophilic inflammation,10 the pattern of response to bronchodilators (BD),11–13 and the findings on imaging studies.
Having taken this previous data into account we decided to investigate the hypothesis that there are more similarities than differences among patients with severe chronic bronchial disease, regardless of whether they have smoking related COPD or bronchiectasis due to other causes.
MethodsPatients who attended our referral outpatient clinic for chronic respiratory failure and whose spirometry showed a pre-BD FEV1<50% of predicted, and FEV1/CVF<70% were invited to participate in this study. We excluded patients with neuromuscular diseases, thoracic deformities, restrictive lung diseases, pulmonary vascular disease and those who had undergone lung resection.
The study was approved by the Ethics Research Committee of our institution and all participants signed an informed consent form.
Clinical data were gathered from patient files. To assess the degree of breathlessness we used the British MRC (Medical Research Council) questionnaire.14 All patients underwent spirometry before and after the use of formoterol 12mcg (Foradil®, Novartis, Brazil). Forced vital capacity (FVC) and slow vital capacity (VC) curves were performed using a flow spirometer (MicroQuark; COSMED SRL, Rome, Italy) with Brazilian reference values.15 The six-minute walk test (6MWT) was performed by the patients who had pulse oxymetry (SpO2) above 90% at rest and the procedure followed that proposed by ATS.16 Body mass index (BMI) was determined and the body composition was assessed by the bioimpedance technique (Biodynamics 310, Seattle, WA, USA). All tests were conducted during periods of clinical stability.
To analyze the data we first compared smokers and non-smokers and secondly we identified two groups according to the pattern of response to BD on spirometry.
When comparing smokers and non-smokers we set parameters and cutoff values for each variable: BMI≤21kg/m2,2,17 MRC>1,17 resting SpO2<90%, use of long-term oxygen therapy (LTOT), FEV1<30% of predicted, inspiratory capacity (IC)<80% predicted, 6MWD<350m,17 and a drop in SpO2 during the 6MWT (SpO2 final–initial)≥4%, which were considered signs of greater severity. For numerical variables [FVC, VC, LM (lean mass) in % of predicted, and lean-to-fat body mass ratio (LM/FM)], we calculated the mean and median of the two groups.
For the analysis of BD response we compared two criteria: ATS/ERS [volume response (FVC) or flow response (FEV1) 200ml and 12%],18 and the parameters used in the study of Paré et al.19 which were applied only when the increase in FEV1 or FVC post-BD was at least 12%. According to Paré et al. the volume response is reflected by the presence of ΔFEV1/ΔCVF<1, and flow response, ΔFEV1/ΔCVF>1, where Δ means the (post-BD−pre-BD) value.19
HRCT of the thorax was performed (sections 1.5–2mm thick, 10mm intervals, at maximum inspiration and expiration)20 and the following CT findings were analyzed: presence of emphysema (centrilobular, paraseptal and panlobular), bronchiectasis, and signs of disease in small airways (air trapping in expiratory scans, tree-in-bud pattern, centrilobular nodules). We also compared the number of lobes affected by bronchiectasis or emphysema.
Variables with normal distribution were analyzed with the Student's t-test. Variables without normal distribution were studied with the Wilcoxon test. Categorical data were compared using Chi-square test or Fisher's exact test when necessary (SAS, version 8, significance with p<0.05).
ResultsOf the 68 patients enrolled, 37 were being followed with a diagnosis of COPD and 31, of extensive bronchiectasis. Patients were diagnosed with COPD if they had chronic respiratory symptoms related to tobacco exposure of at least 10 pack-years (PY), had no history of previous respiratory disease before smoking and showed spirometric findings compatible with the disease; a patient was diagnosed with bronchiectasis if he/she had never smoked or had had a long history of chronic cough and sputum production which preceded the beginning of tobacco use, with the addition of abnormal findings on HRCT (>2 segments with bronchial dilatations).21 The majority of non-smoker patients were diagnosed with bronchiectasis.
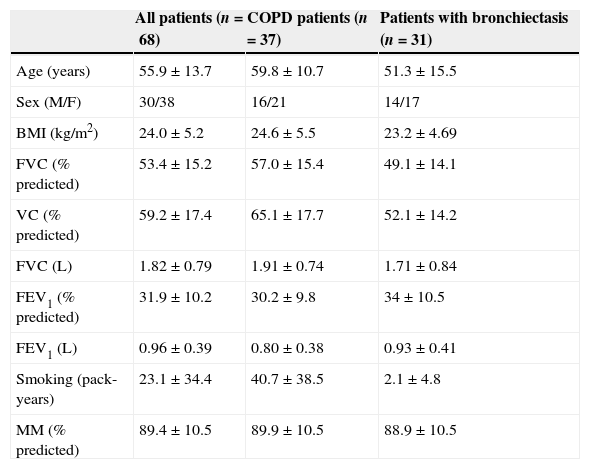
Clinical features, spirometric findings and body composition of the two groups are shown in Table 1.
Clinical features, spirometric findings and body composition of patients.
| All patients (n=68) | COPD patients (n=37) | Patients with bronchiectasis (n=31) | |
|---|---|---|---|
| Age (years) | 55.9±13.7 | 59.8±10.7 | 51.3±15.5 |
| Sex (M/F) | 30/38 | 16/21 | 14/17 |
| BMI (kg/m2) | 24.0±5.2 | 24.6±5.5 | 23.2±4.69 |
| FVC (% predicted) | 53.4±15.2 | 57.0±15.4 | 49.1±14.1 |
| VC (% predicted) | 59.2±17.4 | 65.1±17.7 | 52.1±14.2 |
| FVC (L) | 1.82±0.79 | 1.91±0.74 | 1.71±0.84 |
| FEV1 (% predicted) | 31.9±10.2 | 30.2±9.8 | 34±10.5 |
| FEV1 (L) | 0.96±0.39 | 0.80±0.38 | 0.93±0.41 |
| Smoking (pack-years) | 23.1±34.4 | 40.7±38.5 | 2.1±4.8 |
| MM (% predicted) | 89.4±10.5 | 89.9±10.5 | 88.9±10.5 |
All data are expressed as mean±sd, except sex.
BMI: body mass index; FVC: forced vital capacity; VC: slow vital capacity; FEV1: forced expiratory volume in one second; MM: mean mass.
Among the group with the diagnosis of bronchiectasis, there were ten patients with atypical cystic fibrosis (adults, with late diagnosis and two abnormally elevated sweat chloride concentrations), two with residual lesions from tuberculosis, one with alpha-1-antitrypsin deficiency, one with a history of occupational exposure, and 18 of unknown etiology.
Among 37 COPD patients the CT scans showed some type of emphysema in 29 (78.4%), and bronchiectasis in 18 (48.6%). In the group with the diagnosis of bronchiectasis, 27(87%) had bronchial dilation in more than three lobes and none were found to have emphysema on HRCT scans.
Of 37 COPD patients, 30 were considered current smokers,22 and seven, non-smokers (had never smoked or smoked less than 10 PY). Among the non-smokers, four had diffuse centrilobular emphysema; one patient reported a lifetime of passive smoking; and two patients had panlobular emphysema.
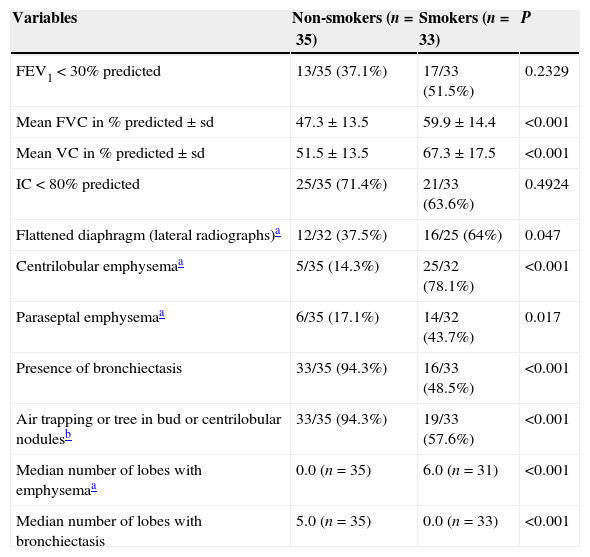
Irrespective of the diagnosis of the COPD, smokers and non-smokers were compared based on their functional and radiological parameters (Table 2). In spirometry, FVC and VC were found significantly smaller in non-smokers (p<0.001), and there was no significant difference between smokers and non-smokers in FEV1 and IC. In the assessment of BMI, MRC, SpO2 at rest, use of LTOT, 6MWD and bioimpedance findings there was no statistically significant difference between groups.
Comparison between non-smokers and smokers.
| Variables | Non-smokers (n=35) | Smokers (n=33) | P |
|---|---|---|---|
| FEV1<30% predicted | 13/35 (37.1%) | 17/33 (51.5%) | 0.2329 |
| Mean FVC in % predicted±sd | 47.3±13.5 | 59.9±14.4 | <0.001 |
| Mean VC in % predicted±sd | 51.5±13.5 | 67.3±17.5 | <0.001 |
| IC<80% predicted | 25/35 (71.4%) | 21/33 (63.6%) | 0.4924 |
| Flattened diaphragm (lateral radiographs)a | 12/32 (37.5%) | 16/25 (64%) | 0.047 |
| Centrilobular emphysemaa | 5/35 (14.3%) | 25/32 (78.1%) | <0.001 |
| Paraseptal emphysemaa | 6/35 (17.1%) | 14/32 (43.7%) | 0.017 |
| Presence of bronchiectasis | 33/35 (94.3%) | 16/33 (48.5%) | <0.001 |
| Air trapping or tree in bud or centrilobular nodulesb | 33/35 (94.3%) | 19/33 (57.6%) | <0.001 |
| Median number of lobes with emphysemaa | 0.0 (n=35) | 6.0 (n=31) | <0.001 |
| Median number of lobes with bronchiectasis | 5.0 (n=35) | 0.0 (n=33) | <0.001 |
FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; VC: slow vital capacity; IC: inspiratory capacity.
HRCT scan findings associated smoking with the presence of centrilobular and paraseptal emphysema (p<0.001 and 0.017, respectively). More lobes were found with bronchiectasis in non-smokers (p<0.001) and more lobes with emphysema in smokers (p<0.001). The signs of small airways disease were more frequently found in non-smokers (p<0.001).
20 patients met the ATS/ERS criteria for volume responders and 7, for flow responders; by Paré et al.19 29 patients could be classified as volume responders and 4 patients were flow responders, respectively. Due to the low number of flow responders, only the characteristics of volume responders and non-responders were compared.
There were 18 patients with FEV1<30% predicted among 29 volume responders and 12 patients with FEV1<30% predicted among 39 without volume response (p=0.010), identified by Paré et al.19 criteria.
The analysis of the other clinical parameters, functional measurements, radiological findings and systemic repercussions showed no statistically significant difference between volume responders and non-responders, regardless of the criteria used.
Patients were not compared by diagnosis, COPD or bronchiectasis.
DiscussionWe included in this study patients with obstructive disease, defined by pre-BD FEV1/FVC<70%, and pre-BD FEV1<50% predicted, with the aim of evaluating severely ill patients. In the literature a European consensus23 provides a precedent for our choice as well as relevant recent studies that have used this criteria.22,24,25
All subjects included in our study had a post-BD FEV1/FVC<70% and a post-BD FEV1<80% predicted, which is in accordance with GOLD diagnostic criteria for COPD.26
Normal spirometry values following BD were an exclusion criterion and therefore asthmatic patients were not included. It was known beforehand that the two diseases that would show up in this series of patients would be COPD and bronchiectasis, which do have several characteristics in common, such as obstruction not fully reversible with BD.
In our study, patients were considered smokers if they had smoked more than 10 PY.22
The comparison of smokers and non-smokers with the same degree of lung function compromise (FEV1) showed that there were few differences between the groups. There was overlap in all clinical and functional parameters, except for VC and FVC which were significantly smaller in non-smokers. When systemic effects were assessed (BMI, the ratio LM/FM and 6MWT) smoking did not affect the results (Table 2).
On HRCT scans, signs of small airway disease were more frequently found in non-smokers (94.3% of the patients, p<0.001). This finding fits in with the hypothesis that bronchiolitis affecting the airways of under 2mm in diameter is a central event in the pathogenesis of bronchiectasis.27
Signs of small airway involvement were also frequent in smokers and were found on HRCT scans in 57.6% of them.
In 1950, Reid27 published a cornerstone study on the pathology of extensive bronchiectasis. One of the most important findings was the reduction of bronchial subdivisions between the hilum and the periphery of the lung. The missing bronchi, whenever their remnants could be identified, were obliterated by fibrous tissue and all the generations of bronchi and small airways that should arise from them had completely disappeared.
Hansell et al.28 suggest that bronchiolitis is the initial lesion in bronchiectasis.
Roberts et al.21 evaluated the presence of airflow obstruction in 100 patients with bronchiectasis and also found that the presence and severity of airflow obstruction correlated with the intensity of mosaic attenuation detected on HRCT.
In patients with clinical diagnosis of COPD in our study bronchiectasis was seen in the scans of 18 subjects (48.6%), a finding that is consistent with the literature in patients of equivalent severity of airway obstruction.29–31
Taking all this into account the hypothesis that smoking related chronic bronchitis and bronchiectasis share many pathogenic events is not farfetched. In fact, according to Boucher,32 there are three well known causes of chronic bronchitis: two of them, cystic fibrosis and primary ciliary diskynesia, are genetically determined, and one is acquired after birth by those individuals who are susceptible, and is most commonly related to tobacco exposure. All three conditions compromise the mucociliary transport apparatus and during their course they all may lead to bronchiectasis.
Considering the pattern of response to bronchodilator in patients with severe obstruction, an important finding was that using both criteria (ATS/ERS18 and Paré et al.19) fewer individuals were considered volume responders by ATS/ERS criteria.
What distinguishes Paré et al.19 criteria is the fact that they do not refer to absolute values; a variation of 12% in FVC and FEV1 is sufficient for the classification of an individual as a volume or flow responder. For patients like the ones in this study, with baseline volumes that are very low, “small” volume variation may represent a large variation in percentage, without reaching the 200ml required by ATS/ERS.18
As expected, flow response was not significant in our sample of patients with severe bronchial obstruction.
In addition to detecting a larger number of volume responder patients, Paré et al.19 criteria allowed the demonstration of an association between the severity of obstructive disease, as assessed by FEV1, and the volume response (18 patients with FEV1<30% among 29 volume responders and 12 patients with FEV1<30% among 39 without volume response, p=0.010).
Schermer et al.12 analyzing BD response in 2210 COPD patients found a positive correlation between ΔFEV1 and ΔCVF with each stage: ΔFEV1 declined toward the most severe GOLD stages, while ΔCVF augmented. These findings are in agreement with ours, provided that Paré et al.19 criteria are applied to the sample.
ConclusionsIn patients with severe obstruction the smoking etiology does not appear to be relevant in determining systemic complications such as hypoxemia, exercise capacity and loss of lean body mass. Bronchiectasis was found in a significant proportion of patients with severe COPD.
Volume response is more common than flow response in patients with severe obstruction. The comparison of two criteria for assessing bronchodilator response showed that Paré et al. criteria are more sensitive than ATS/ERS criteria in identifying volume response in this subgroup of patients. Further studies with a larger number of patients might confirm our findings.
Conflicts of interestThe authors have no conflicts of interest to declare.