The aim of our study is to find differences in IgG in sera of potentially exposed and nonexposed individuals and to detect differences in concentrations of specific serum IgG among subjects with and without EAA.
Seventy-two patients being followed for suspected interstitial lung disease were included. Specific IgG in sera were established by ImmunoCAP.
Serum concentrations of Aspergillus fumigatus, Candida albicans IgG and mixture of moulds IgG were higher in subjects with exposure to relevant inhalation antigens (p<0.05). Patients exposed to parrot and mammal hair mixture had higher serum concentration of specific IgG (p<0.05). Subjects without exposure to mites had lower serum IgG to Dermatophagoides pteronyssinus, Dermatophagoides farinae, Dermatophagoides microceras and Glycophagus domesticus (p<0.05).
Higher concentration of serum specific IgG may show previous exposure to this antigen. Even though mite specific IgG are not commonly tested in EAA patients, we suggest their immunomodulatory activity may influence susceptibility to other inhalation antigens.
O objetivo do nosso estudo é descobrir diferenças da IgG no soro de possíveis indivíduos expostos e não-expostos, bem como detetar diferenças de concentrações da IgG sérica específico entre indivíduos com e sem AAE.
Foram incluídos setenta e dois pacientes com suspeita de doença pulmonar intersticial. A IgG sérica específica foi definida pelo ImmunoCAP.
As concentrações séricas de IgG para o Aspergillus fumigatus, Candida albicans e mistura de fungos foram superiores em sujeitos expostos a inalação de antigénios relevantes (p<0,05). Os pacientes expostos a uma mistura de penas de papagaio e pelos de mamíferos apresentaram uma maior concentração sérica da IgG específica (p<0,05). Os indivíduos sem exposição a ácaros apresentaram menor IgG sérica para Dermatophagoides pteronyssinus, D. farinae, D. microceras e Glycophagus domesticus (p<0,05).
Uma elevada concentração de IgG sérica específica pode indicar uma exposição prévia a este antigénio. Embora a IgG específica para os ácaros não seja normalmente testada em pacientes com AAE, referimos que a sua atividade imunomoduladora pode influenciar a suscetibilidade de outras inalações de antigénios.
Extrinsic allergic alveolitis (EAA) is an interstitial lung disease which develops in susceptible individuals after repeated exposure to inhalation antigens. Many antigens are capable of provoking EAA. The best studied forms of EAA include farmer's lung, summer-type pneumonitis and pigeon breeder's disease. Recent reports suggest that the household environment is a potent source of antigens.1 EAA following exposure to feather duvets, steam iron, or cat fur has been documented.2–4 The spectrum of antigens varies not only with environment but also with different geographical locations.5 The immune response to inhalation antigens can be detected by production of serum specific immunoglobulin G (IgG).6 The diagnostic value of specific serum IgG in EAA patients is controversial. No physiological ranges for specific IgG against possible causative antigens have been established for the Czech population. The aim of our study is to discover whether there are any differences in specific IgG in sera of potentially repeatedly exposed and nonexposed individuals and above all to detect possible differences in concentrations of specific serum IgG among subjects with subacute EAA, chronic EAA and subjects without EAA.
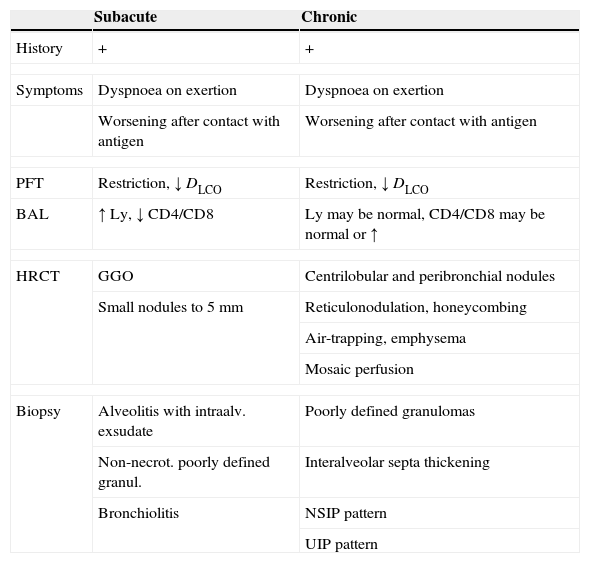
Materials and methodsStudy populationSeventy-two patients being followed at the Department of Respiratory Diseases for suspected interstitial lung disease (between 2008 and 2010) were included in the study. The research process consisted of a detailed history of exposure, clinical investigation, laboratory tests including assessment of specific serum IgG and concentration of total serum IgG, lung function tests, bronchoscopy with bronchoalveolar lavage, cytology and flow cytometry of the recovered fluid (BALF), transbronchial biopsy and high resolution tomography of the chest (HRCT). There are no widely accepted diagnostic criteria for subacute and chronic EAA. The diagnostic criteria used at our Department are outlined in Table 1.
Diagnostic criteria for EAA used at the Department of Respiratory Diseases, Thomayer's University Hospital, Prague.
| Subacute | Chronic | |
|---|---|---|
| History | + | + |
| Symptoms | Dyspnoea on exertion | Dyspnoea on exertion |
| Worsening after contact with antigen | Worsening after contact with antigen | |
| PFT | Restriction, ↓ DLCO | Restriction, ↓ DLCO |
| BAL | ↑ Ly, ↓ CD4/CD8 | Ly may be normal, CD4/CD8 may be normal or ↑ |
| HRCT | GGO | Centrilobular and peribronchial nodules |
| Small nodules to 5mm | Reticulonodulation, honeycombing | |
| Air-trapping, emphysema | ||
| Mosaic perfusion | ||
| Biopsy | Alveolitis with intraalv. exsudate | Poorly defined granulomas |
| Non-necrot. poorly defined granul. | Interalveolar septa thickening | |
| Bronchiolitis | NSIP pattern | |
| UIP pattern | ||
BAL, bronchoalveolar lavage; DLCO, diffusing capacity; Ly, lymphocytes; GGO, ground glass opacity; HRCT, high resolution computed tomography; NSIP, nonspecific interstitial pneumonitis; PFT, pulmonary function tests; UIP, usual interstitial pneumonitis.
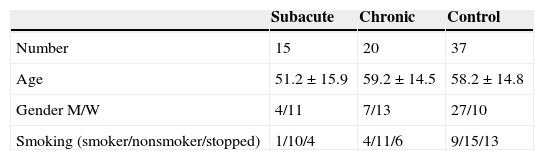
After completing the diagnostic programme, 15 subjects matched a diagnosis of subacute EAA, 20 were diagnosed as chronic EAA and the other 37 patients formed a control group.
The control group consisted of 6 patients with nonspecific interstitial pneumonia, 10 with idiopathic pulmonary fibrosis, 4 had organising pneumonia, 7 chronic form of sarcoidosis, 3 carcinomatous lymfangitis and 7 were diagnosed with interstitial pneumonia. Serum concentrations of specific IgG were not used either for confirmation or for rejection of definite diagnosis.
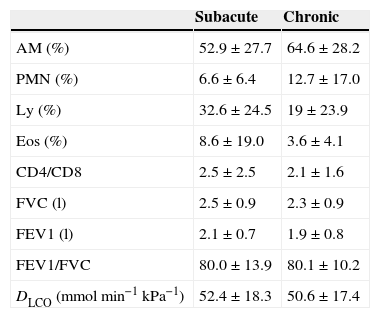
Demographic data of the study group are included in Table 2. BAL and pulmonary function tests data are summarized in Table 3.
Bronchoalveolar lavage results and pulmonary function test data in subacute and chronic EAA groups.
| Subacute | Chronic | |
|---|---|---|
| AM (%) | 52.9±27.7 | 64.6±28.2 |
| PMN (%) | 6.6±6.4 | 12.7±17.0 |
| Ly (%) | 32.6±24.5 | 19±23.9 |
| Eos (%) | 8.6±19.0 | 3.6±4.1 |
| CD4/CD8 | 2.5±2.5 | 2.1±1.6 |
| FVC (l) | 2.5±0.9 | 2.3±0.9 |
| FEV1 (l) | 2.1±0.7 | 1.9±0.8 |
| FEV1/FVC | 80.0±13.9 | 80.1±10.2 |
| DLCO (mmolmin−1kPa−1) | 52.4±18.3 | 50.6±17.4 |
Results are expressed as mean±SD. AM, alveolar macrophage; DLCO, diffusing capacity; Eos, eosinophil; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; Ly, lymphocyte; PMN, polymorphonuclear cell.
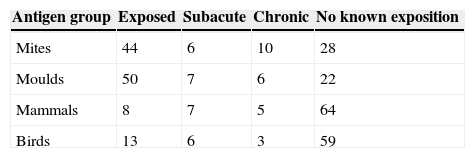
Exposure history was taken for each investigated group of antigens (mites, moulds, mammals and birds). Subjects were divided into exposed and nonexposed individuals according to the their clinical history.
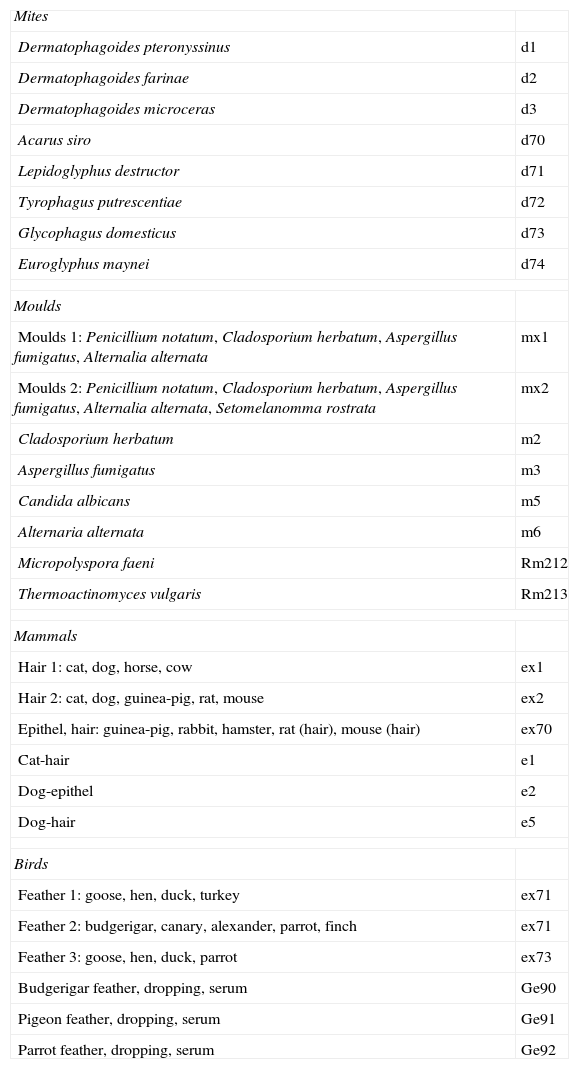
Assessment of specific IgG in seraSpecific IgG in sera of examined subjects were established by ImmunoCAP (Phadia) according to the manufacturer's instructions. Table 4 shows the spectrum of investigated specific IgG.
Spectrum of investigated specific serum IgG.
| Mites | |
| Dermatophagoides pteronyssinus | d1 |
| Dermatophagoides farinae | d2 |
| Dermatophagoides microceras | d3 |
| Acarus siro | d70 |
| Lepidoglyphus destructor | d71 |
| Tyrophagus putrescentiae | d72 |
| Glycophagus domesticus | d73 |
| Euroglyphus maynei | d74 |
| Moulds | |
| Moulds 1: Penicillium notatum, Cladosporium herbatum, Aspergillus fumigatus, Alternalia alternata | mx1 |
| Moulds 2: Penicillium notatum, Cladosporium herbatum, Aspergillus fumigatus, Alternalia alternata, Setomelanomma rostrata | mx2 |
| Cladosporium herbatum | m2 |
| Aspergillus fumigatus | m3 |
| Candida albicans | m5 |
| Alternaria alternata | m6 |
| Micropolyspora faeni | Rm212 |
| Thermoactinomyces vulgaris | Rm213 |
| Mammals | |
| Hair 1: cat, dog, horse, cow | ex1 |
| Hair 2: cat, dog, guinea-pig, rat, mouse | ex2 |
| Epithel, hair: guinea-pig, rabbit, hamster, rat (hair), mouse (hair) | ex70 |
| Cat-hair | e1 |
| Dog-epithel | e2 |
| Dog-hair | e5 |
| Birds | |
| Feather 1: goose, hen, duck, turkey | ex71 |
| Feather 2: budgerigar, canary, alexander, parrot, finch | ex71 |
| Feather 3: goose, hen, duck, parrot | ex73 |
| Budgerigar feather, dropping, serum | Ge90 |
| Pigeon feather, dropping, serum | Ge91 |
| Parrot feather, dropping, serum | Ge92 |
Results are expressed as mean±standard deviation (SD). Kruskal–Wallis was used to compare concentrations of specific IgG. p values <0.05 were regarded as significant.
ResultsThe numbers of patients in groups according to their history of exposure are included in Table 5. Possible occupational exposure was detected in 10 patients (exposure to bird antigens in 2 cases, mammals 5 cases, moulds 3 cases). Sixteen patients reported farming activities in their free time, 8 were exposed to bird antigens, 5 to mammals and 4 to moulds. Forty-four patients indicated household exposure – 11 kept birds, 12 mammals, 15 were exposed to moulds and 6 to dust and possibly mites.
Possible occupational exposure was detected in 7 EAA patients (bird antigens in one case, mammals in four cases and moulds 4 times). Two patients with EAA were involved in farming activities with exposure to bird antigens. Nine EAA patients kept birds, 11 kept a mammal at home, six claimed exposure to moulds at home and five reported a dusty environment. Seven EAA patients had more than one possible source of antigen inhalation.
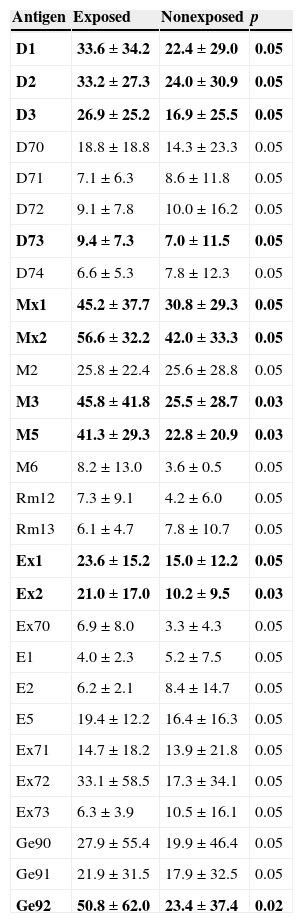
Serum concentrations of total IgG were within normal limits (8–13.5g/l) in all examined individuals. Serum concentration of Aspergillus fumigatus, Candida albicans, moulds 1 and 2 IgG was significantly higher in subjects with confirmed exposure to relevant inhalation antigens (p<0.05). Patients exposed to parrots (Ge92) and hair 1, 2 (ex1, ex2) had a significantly higher serum concentration of specific IgG than patients with no documented exposure (p<0.05). Subjects who had taken precautions to reduce their exposure to mites (bedding made of hollow fibres, no carpets, no curtains, etc.) had lower serum IgG to Dermatophagoides pteronyssinus, Dermatophagoides farinae, Dermatophagoides microceras and Glycophagus domesticus than the rest of examined individuals (p<0.05). Concentrations of specific IgG in serum of exposed and nonexposed individuals are summarized in Table 6.
Concentrations of specific IgG in sera of exposed and nonexposed individuals.
| Antigen | Exposed | Nonexposed | p |
|---|---|---|---|
| D1 | 33.6±34.2 | 22.4±29.0 | 0.05 |
| D2 | 33.2±27.3 | 24.0±30.9 | 0.05 |
| D3 | 26.9±25.2 | 16.9±25.5 | 0.05 |
| D70 | 18.8±18.8 | 14.3±23.3 | 0.05 |
| D71 | 7.1±6.3 | 8.6±11.8 | 0.05 |
| D72 | 9.1±7.8 | 10.0±16.2 | 0.05 |
| D73 | 9.4±7.3 | 7.0±11.5 | 0.05 |
| D74 | 6.6±5.3 | 7.8±12.3 | 0.05 |
| Mx1 | 45.2±37.7 | 30.8±29.3 | 0.05 |
| Mx2 | 56.6±32.2 | 42.0±33.3 | 0.05 |
| M2 | 25.8±22.4 | 25.6±28.8 | 0.05 |
| M3 | 45.8±41.8 | 25.5±28.7 | 0.03 |
| M5 | 41.3±29.3 | 22.8±20.9 | 0.03 |
| M6 | 8.2±13.0 | 3.6±0.5 | 0.05 |
| Rm12 | 7.3±9.1 | 4.2±6.0 | 0.05 |
| Rm13 | 6.1±4.7 | 7.8±10.7 | 0.05 |
| Ex1 | 23.6±15.2 | 15.0±12.2 | 0.05 |
| Ex2 | 21.0±17.0 | 10.2±9.5 | 0.03 |
| Ex70 | 6.9±8.0 | 3.3±4.3 | 0.05 |
| E1 | 4.0±2.3 | 5.2±7.5 | 0.05 |
| E2 | 6.2±2.1 | 8.4±14.7 | 0.05 |
| E5 | 19.4±12.2 | 16.4±16.3 | 0.05 |
| Ex71 | 14.7±18.2 | 13.9±21.8 | 0.05 |
| Ex72 | 33.1±58.5 | 17.3±34.1 | 0.05 |
| Ex73 | 6.3±3.9 | 10.5±16.1 | 0.05 |
| Ge90 | 27.9±55.4 | 19.9±46.4 | 0.05 |
| Ge91 | 21.9±31.5 | 17.9±32.5 | 0.05 |
| Ge92 | 50.8±62.0 | 23.4±37.4 | 0.02 |
Results are expressed as mean±SD (mg/l). p=statistically significant difference. In bold: p < 0.05 were regarded as significant.
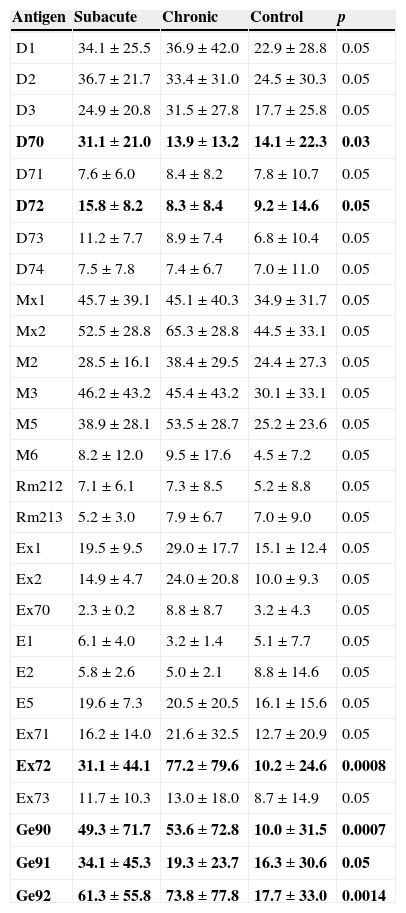
Concentrations of specific IgG in serum of control group, patients with subacute EAA and chronic EAA are summarized in Table 7.
Concentrations of specific IgG in sera of subacute and chronic EAA patients and control group.
| Antigen | Subacute | Chronic | Control | p |
|---|---|---|---|---|
| D1 | 34.1±25.5 | 36.9±42.0 | 22.9±28.8 | 0.05 |
| D2 | 36.7±21.7 | 33.4±31.0 | 24.5±30.3 | 0.05 |
| D3 | 24.9±20.8 | 31.5±27.8 | 17.7±25.8 | 0.05 |
| D70 | 31.1±21.0 | 13.9±13.2 | 14.1±22.3 | 0.03 |
| D71 | 7.6±6.0 | 8.4±8.2 | 7.8±10.7 | 0.05 |
| D72 | 15.8±8.2 | 8.3±8.4 | 9.2±14.6 | 0.05 |
| D73 | 11.2±7.7 | 8.9±7.4 | 6.8±10.4 | 0.05 |
| D74 | 7.5±7.8 | 7.4±6.7 | 7.0±11.0 | 0.05 |
| Mx1 | 45.7±39.1 | 45.1±40.3 | 34.9±31.7 | 0.05 |
| Mx2 | 52.5±28.8 | 65.3±28.8 | 44.5±33.1 | 0.05 |
| M2 | 28.5±16.1 | 38.4±29.5 | 24.4±27.3 | 0.05 |
| M3 | 46.2±43.2 | 45.4±43.2 | 30.1±33.1 | 0.05 |
| M5 | 38.9±28.1 | 53.5±28.7 | 25.2±23.6 | 0.05 |
| M6 | 8.2±12.0 | 9.5±17.6 | 4.5±7.2 | 0.05 |
| Rm212 | 7.1±6.1 | 7.3±8.5 | 5.2±8.8 | 0.05 |
| Rm213 | 5.2±3.0 | 7.9±6.7 | 7.0±9.0 | 0.05 |
| Ex1 | 19.5±9.5 | 29.0±17.7 | 15.1±12.4 | 0.05 |
| Ex2 | 14.9±4.7 | 24.0±20.8 | 10.0±9.3 | 0.05 |
| Ex70 | 2.3±0.2 | 8.8±8.7 | 3.2±4.3 | 0.05 |
| E1 | 6.1±4.0 | 3.2±1.4 | 5.1±7.7 | 0.05 |
| E2 | 5.8±2.6 | 5.0±2.1 | 8.8±14.6 | 0.05 |
| E5 | 19.6±7.3 | 20.5±20.5 | 16.1±15.6 | 0.05 |
| Ex71 | 16.2±14.0 | 21.6±32.5 | 12.7±20.9 | 0.05 |
| Ex72 | 31.1±44.1 | 77.2±79.6 | 10.2±24.6 | 0.0008 |
| Ex73 | 11.7±10.3 | 13.0±18.0 | 8.7±14.9 | 0.05 |
| Ge90 | 49.3±71.7 | 53.6±72.8 | 10.0±31.5 | 0.0007 |
| Ge91 | 34.1±45.3 | 19.3±23.7 | 16.3±30.6 | 0.05 |
| Ge92 | 61.3±55.8 | 73.8±77.8 | 17.7±33.0 | 0.0014 |
Results are expressed as mean±SD (mg/l). p=statistically significant difference. In bold: p < 0.05 were regarded as significant.
While assessing antibody response to inhalation antigens in EAA patients, it is important to choose relevant antigens. This study is the first one to test a broad spectrum of response to antigens; a spectrum of selected antigens and differences in specific serum IgG concentrations between exposed, nonexposed and EAA populations are further discussed. Most of the subjects in the group reported exposure to inhalation antigens in their household environment. We can assume that exposure to inhalation antigens in a household environment is continuous, which makes the presence of specific IgG against the offending antigen more likely.7,8 Antigens of moulds are ubiquitous – particularly the investigated species (Aspergillus sp., Cladosporium sp., Alternaria sp. and Penicillium sp.) which are commonly detected in damp buildings in central Europe.9,10Candida sp. is not usually detected in samples of house dust, but it can be found in oral or gut fungal microbiofilm in healthy individuals and its role in developing EAA in farm workers has been documented.11–13 EAA induced by endogenous C. albicans was observed as well.14
We found a significantly higher serum concentration of specific IgG to mixtures of antigens moulds 1 and 2 in exposed individuals (Table 4). A significant difference was observed also in A. fumigatus and C. albicans IgG concentrations between exposed and nonexposed subjects. No difference in serum concentrations of moulds antibodies in EAA patients and subjects without EAA was found. We conclude that in individuals with household exposure to moulds, concentrations of specific serum IgG will not help us to confirm diagnosis of EAA.
Some of our patients also had a history of occupational exposure to moulds. They were also tested for Micropolyspora sp. and Thermoactinomyces sp. serum specific IgG, because both of these have been detected as leading to antigens of farmer's lung in Finland, India, China, Spain.15 Antigens causing EAA in Czech farmers have not been identified, although the relevance of tested antigens has been highlighted by various authors.16 Since no differences in serum anti-Micropolyspora sp. and anti-Thermoactinomyces sp. IgG concentration between exposed and nonexposed subjects were found, we suggest these moulds may not trigger farmer's lung disease in Czech farmers. Similarly, neither Micropolyspora sp. nor Thermoactinomyces sp. were found in Switzerland, Canada or United States,17 perhaps due to different geographical conditions.
Mites as well as moulds are ubiquitous organisms and exposure history data are difficult to obtain. Exposure occurs not only at home (a dusty household environment was taken into account) but also in the farming population and among bird keepers.18,19 Although no case of EAA provoked by exposure to mites has been described, EAA developed after exposure to insect proteins is well known (miller's lung, lycoperdonosis). Six patients in our study group had a history of exposure to dust in the home. The panel of investigated antigens (Table 4) included D. pteronyssinus, D. farinae, D. microcerans and Euroglyphus maynei, which are known to be house dust mites. Concentration of specific IgG for D. pteronyssinus, D. farinae and D. microceras was found to be higher in serum of exposed subjects than in nonexposed individuals. Furniture mites G. domesticus feed on moulds and the association between G. domesticus and the occurrence of moulds is likely. Patients exposed to house dust had higher serum concentration of specific IgG against this mite than those not exposed. The observation was not supported by different serum IgG concentrations in EAA vs. control group. The farming population might be exposed to so-called storage mites: Acarus siro, Lepidoglyphus destructor and Tyrophagus putrescentiae. A. siro occurs as an agricultural pest, T. putrescentiae is a pest in many foods including dog food. L. destructor can be found in animal food, it consumes moulds, mostly Alternaria sp. and Penicillium sp. We found a significantly higher serum IgG concentration against A. siro and T. putrescentiae in patients with subacute EAA and a corresponding exposure history than in the patients with chronic EAA and the control group. We suggest that mites’ antigens should be considered as possible provoking agents in subjects with a history of exposure to dust and moulds who have suspected EAA. Particles of mites smaller than 5μm can penetrate the distal respiratory tract and may be able to start an immune response leading to EAA. Moreover, since mites antigens provide strong proteinase activity, they might modify the immune response to other inhaled antigens.20 Brown et al. showed that major antigen of D. pteronyssinus – Der p1 – can modulate the adaptive immune system by cleavage of CD23 and CD25, which may promote an allergic inflammatory response.21 Further, Der p1 disrupts epithelial tight junctions, which facilitates the transport of allergen across the epithelium. Der p1 and other mite antigens cause release of proinflammatory cytokines from bronchial epithelial cells, mast cells and basophils.22 They cause degradation of surfactant proteins SP-A and SP-D.23 Surfactant properties of SP-A and SP-B were found to be changed in EAA patients.24 Higher serum levels of SP-D in EAA patients with severe lung function impairment and better recovery over time documented by Jansen et al. may be the consequence of more rapid surfactant turnover.25
Furrier's lung and animal handler's lung are caused by animal fur dust, proteins, urine, serum and pelts of rats and gerbils.26 Even though exposure to animal fur, serum and secretions could be expected in farmers and pet keepers, we found no report in the literature about the measurement of the specific serum IgG concerned. We found a significantly increased serum IgG against a mixture of antigens hair 1 and hair 2 in exposed individuals compared to subjects without a history of exposure to these antigens. We found no differences between serum concentrations of antibodies against animal antigens in EAA patient and the control group, which may discourage use of these specific IgG in to confirm diagnosis.
Bird fancier's lung is one of the best studied and most frequent forms of EAA. Several studies have aimed to quantify serum antibodies in this form of EAA. The incidence of serum specific IgG in asymptomatic bird keepers is 40%.7,8 McSharry et al. proposed that the presence of serum antibodies may reflect sub-clinical inflammation, but no studies involving longitudinal monitoring of specific IgG concentration in serum of exposed individuals are available.27 Patients, who were exposed to avian inhalation antigens in their childhood or during later life, may develop feather duvet lung after exposure to bedding filled with goose feathers.28 We documented higher concentration of specific IgG against mixture of parrot derived antigens in exposed individuals than in the nonexposed group. Generally, except for mixture of antigens Feather 1, significantly higher serum IgG concentrations of all groups of bird antigens were observed in subacute EAA patients compared to the control group. Patients with chronic EAA tested for Feather 3 and budgerigar had higher appropriate specific IgG serum concentrations than the control group.29,30
It is unlikely that patients with EAA are exposed to just a single inhalation antigen. Farming population can be exposed to moulds, mites, avian proteins and animal's fur, serum and urine. Thus it may be difficult to differentiate trigger antigens.
ImmunoCAP technology was used to measure specific serum IgG concentrations. Previous studies have proved that results achieved by ImmunoCAP method are consistent with precipitation technique.31 It could be argued that the control group did not consist of healthy volunteers, and patients with various interstitial lung diseases were involved. Yoshizawa et al. suggested that preexisting inflammatory disease alters the antibody response to inhaled antigens and that modulation of antibody response is dependent on the preexisting condition. We find that the enrolment of patients with interstitial lung diseases other than EAA as a control group is useful not only as a control group per se but also from the differential diagnostic point of view.32
We conclude that concentrations of serum IgG against moulds and mammals antigens does not differentiate between exposure and EAA disease. On the other hand, higher serum concentrations of specific IgG against mites and avian antigens are found in exposed individuals as well as in subacute and chronic EAA patients. These two antigen groups and the antibody response to them should be further studied, because they can help us to clarify the pathogenesis of EAA and can serve as useful diagnostic tools. Finding higher specific IgG concentrations should lead to a detailed examination of the patient's history with particular reference to inhalation exposure. Detection and elimination of the source of inhalation antigen is also one of the steps in the treatment of EAA patients. Only relevant specific IgG should be tested, as documented by our research into mould specific IgG concentrations in the Czech farming population.
Conflicts of interestThe authors have no conflicts of interest to declare.