Dear Editor,
Diagnosis of alpha-1 antitrypsin deficiency (AATD) had improved considerably due to an increasing awareness about the disease and the publication of evidence-based international diagnostic and management guidelines in the last 15 years.1,2
Patients with AATD are at increased risk of developing lung disease, like panlobular basal emphysema, chronic obstructive pulmonary disease (COPD) and bronchiectasis.1,3,4 On the other hand, liver fibrosis, steatosis, cirrhosis and hepatocarcinoma had also been described in AATD patients.1,4 Pi*ZZ is the most frequent genotype associated with both pulmonary and hepatic manifestations of AATD,2,4 affecting approximately 1/3500 to 1/6000 individuals of European descent.5 Other rare deficiency variants were described worldwide, like MMalton, (rs775982338: p.Phe76del) and several null variants (Q0), the latter being unable to produce AAT and to form polymers in the liver.1,4
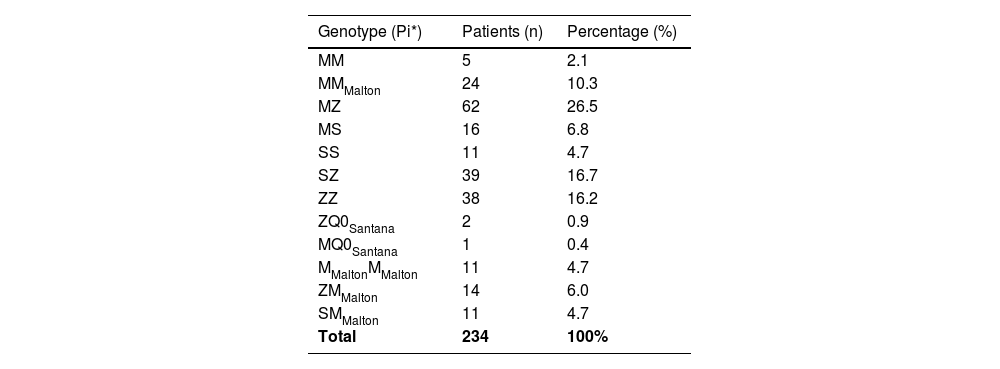
Madeira Island has a population of 250.000 people and has been described as having one of the highest prevalence of patients with AATD.6 Here, we report the frequency of AATD patients on Madeira Island, tested with a clinical suspicion or through family screening, followed by genotyping, with AAT serum level below 110 mg/dL.1 Currently, 274 patients are diagnosed with AATD in our hospital, of whom 234 have had the complete genetic study (Table 1). The average AAT concentration value is 58.4 (13.5–108.0) mg/dL. The Pi*Z allele is present in 66.3% of patients, but we also note the high frequency of a rare variant not previously mentioned in Spínola C et al. paper,6 the MMalton, seen in 25.7% of patients (4.7% in homozygosity).
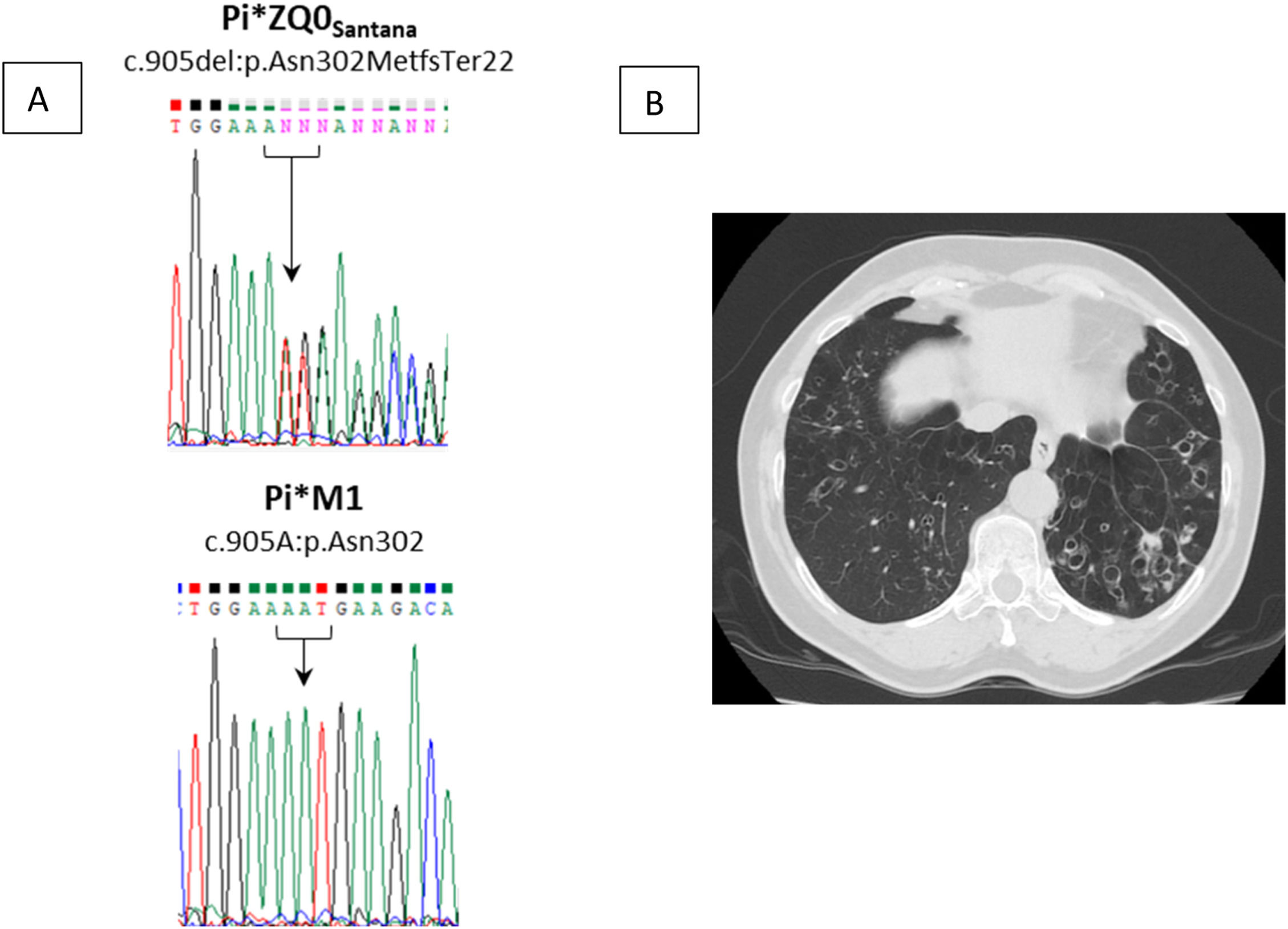
Moreover, we recently identified a novel null allele named Pi*Q0Santana (NM_000295.5:c.905del:p.Asn302MetfsTer22) that is absent from public genomics databases (Fig. 1A). This was detected in a family from Santana city, in the Northeast region of Madeira Island. The index case is a 61-year-old woman, non-smoker, with a medical history of recurrent respiratory infections and bronchiectasis diagnosed at age 36. A few years later, her blood analysis showed reduced AAT levels (13.5 mg/dL) and the genetic screening revealed a Pi*MZ genotype. Given that the serum levels were not in accordance with the genotype, raising the suspicion of a rare allele, a patient sample was sent to a reference laboratory. The second analysis uncovered a Pi*ZQ0Santana genotype. Her sister, a 72-year-old woman, had a similar history: Pi*MZ genotype associated with serum level of 17.2 mg/dL. A later diagnosis performed more recently matched with Pi*ZQ0Santana as well. They have 2 brothers who also have AATD, a 70-year-old man, non-smoker, with Pi*ZZ (18.4 mg/dL) and a 67-year-old man, ex-smoker, with Pi*MQ0Santana (66.8 mg/dL). At present, all show airflow obstruction compatible with COPD, emphysema and chronic Pseudomonas aeruginosa infection. Curiously, in the last two years, Mycobacterium avium complex was isolated from two sputum samples of the index case. The two oldest siblings are currently under long-term oxygen therapy and, except for the Pi*MQ0Santana, the remaining 3 siblings have been receiving AAT augmentation therapy for at least 5 years. In the index case, AAT therapy seemed to stabilize progression of emphysema or bronchiectasis, with an improvement in mucoid impaction as reported in a recently performed thoracic computed tomography (Fig. 1B).
There are two important criteria defined for initiation of treatment with augmentation therapy: genotypes with two alleles that cause severe disease plus a serum level of AAT ≤ 57 mg/dL (as determined by nephelometry), and lung function impairment.1,2 Patients should be non-smokers or ex-smokers (≥ 6 months).1-3 Three patients of this family of four siblings met these criteria. With augmentation therapy, prevention of emphysema progression and improvement of quality of life were observed. NB, patients with selective immunoglobulin A (IgA) deficiency should not receive augmentation therapy, because this treatment contains small amounts of IgA which can lead to adverse anaphylactic reactions.1,2 Curiously, in Madeira Island we previously identified a rare case of Common Variable Immunodeficiency (CVID) combined with severe AATD, who is receiving the two replacement therapies, with no adverse reactions so far.7 Nonetheless, each case must be evaluated individually.
We observed that the prevalence of certain alleles has been increasing in Madeira population, like Pi*Z and Pi*S. Previously, in a paper focused on this subject, 8 patients with Pi*MZ, 6 with Pi*SS, 2 with Pi*SZ and no Pi*ZZ patients were reported.6 Nowadays, we have identified an increase in the frequency of those genotypes: 62, 11, 39 and 38 patients, respectively. In addition, rare deficiency and null variants were detected accounting for a significant percentage of the patients followed in our hospital, namely MMalton (n = 60; 25,6%) and Q0Santana (n = 3; 1,3%).
AATD´s diagnosis is increasing in Madeira Island and severe disease is seen in many patients, due to the high prevalence of Pi*Z and Pi*MMalton. Recently we discovered a null variant specific of this region, Q0Santana. Even in non-smokers, the presence of these alleles is responsible for remarkable lung parenchyma alterations and impaired lung function.