Crewmembers overwintering at Concordia Station in Antarctica are exposed to daylight alterations, hypobaric hypoxia, confinement and cold. Previous studies reported persistent sleep disturbances, sleep-disordered breathing and reduced daytime performance over a period of 12 months but consequences on the cardiovascular function remain unknown.1,2 Concordia is located at 3233 m of altitude and non-Antarctic altitude studies have shown acute altitude-induced increases in systemic blood pressure (BP) up to several weeks.3,4 Revealing persistent cardiovascular stress, e.g. elevated incidence of day-to-night BP non-dipping,5 in Concordia crewmembers over a period of 12 months would call for immediate actions to identify risk factors and investigate prophylactic measures that can prevent adverse cardiovascular events in future expeditions.
Therefore, the main purpose of this study was to prospectively investigate cardiovascular function in the presence of sleep disturbances in crewmembers staying for 12 months at Concordia. Secondary, to distinguish the effect of altitude from other Antarctic conditions (e.g., daylight alterations), observations in Concordia were compared to a low-altitude control group stationed for 12 months at Dumont d'Urville (DdU), located on the Antarctica coast.
This study was approved by a French ethical committee (CPP Nord-Ouest 1, no.19.02.28.36850). Healthy crewmembers without any chronic disease or intake of regular medication, living ≤1200 m and who gave their written consent performed baseline sea-level measurements in France 5–6 weeks before embarking on their 12-month mission at Concordia (3233 m, barometric pressure 645 hPa, corresponding to ∼3800 m at the latitude of 45° in the Northern hemisphere) or at DdU (20 m, 985 hPa). No pre-acclimatization to high altitude was performed. During baseline measurements and in the 1st and 12th month in Antarctica, participants underwent 24-hour ambulatory blood pressure (BP) monitoring (24h-ABPM, Novacor Diasys 3+, France) according to international standards5 and sleep stage assessments for 2 consecutive nights using a DREEM headband (Dreem 1, France). 24h-ABPM was analysed by linear mixed regression analyses incorporating all valid BP measurements (n = 4181) obtained during the three 24h-ABPM sessions (measurements were performed in 15 and 30 min intervals during day and night, respectively). Night periods were adjusted to individual habits and were detected by the position sensor of the BP device when a participant was in supine position for at least two consecutive measurements. To detect clinically relevant nocturnal (abnormal) non-dippers, non-dipping was defined by a <10% decrease from day-to-night BP.5 Sleep outcomes with a confidence quality score of >50% were automatically scored by the DREEM algorithm.6 A P < 0.05 was considered as statistically significant.
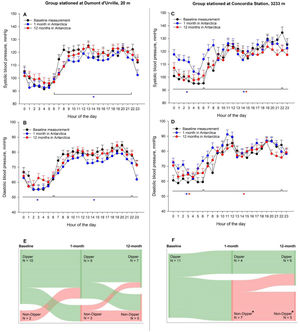
Overall, 12 healthy crewmembers (17% women, mean±SD age 30.6 ± 11.7 yrs) at DdU and 11 (36% women, age 36.2 ± 10.0 yrs) at Concordia (P = 0.129 between groups) participated in the study. All tolerated the stay in Antarctica and no regular medication was used against altitude illnesses or to facilitate sleep. At both Stations, indoor temperature was around 22 °C. Crewmembers at Concordia were hypoxemic but showed improved sleep onset latency and sleep efficiency during the 1st and 12th month compared to baseline. However, they spent a higher proportion of total sleep time (TST) in superficial sleep stage 2 and a lower proportion in deep sleep stage 3 compared to baseline (Table). More micro-arousals were observed during the 1st and 12th month at Concordia compared to baseline and DdU. No changes in sleep stages and micro-arousals were observed at DdU. At Concordia, crewmembers showed elevated night BP values in the 1st month compared to baseline and DdU (Table, Figure Panel A to D), whereas nocturnal diastolic and mean BP elevations persisted at 12 months. Additionally, the proportion of non-dippers in Concordia increased from 0% at baseline to 64% and 45% in the 1st and 12th month, respectively (P<0.05 vs. baseline, McNemar Tests) (Figure, Panel E and F). Corresponding proportions of non-dippers in DdU were 17%, 25% and 42% (P>0.05, all comparisons).
Sleep- and ambulatory blood pressure-related outcomes.
| Dumont D'Urville Station (20 m) | Concordia Station (3233 m) | Mean difference in change between groups (95% CI) | ||||||
| Exposure time, months | Baseline | 1 | 12 | Baseline | 1 | 12 | 1 month vs. baseline | 12 month vs. baseline |
| Daytime SpO2,% | 97.6±0.5 | 97.6±0.5 | 98.0±0.5 | 96.6±0.5 | 89.7±0.5⁎ | 90.6±0.5⁎ | −7.0 (−8.2 to −5.7)‡ | −6.5 (−7.7 to −5.2)‡ |
| Sleep-related outcomes | ||||||||
| Time in bed, min | 400±23 | 436±17 | 387±18 | 434±21 | 438±15 | 458±15 | −32 (−109 to 45) | 37 (−22 to 95) |
| TST, min | 357±24 | 391±19 | 344±20 | 358±20 | 384±17 | 408±16 | −8 (−88 to 72) | 64 (−5 to 133) |
| Sleep onset latency, min | 15±5 | 15±4 | 15±4 | 32±4 | 19±4⁎ | 17±4⁎ | 13 (−27 to 1) | 15 (−30 to 1) |
| N1, %TST | 5.3±0.8 | 5.0±0.6 | 5.8±0.7 | 5.9±0.7 | 6.5±0.6 | 6.7±0.6 | 0.9 (−1.5 to 3.3) | 0.3 (−2.2 to 2.8) |
| N2, %TST | 38.4±3.0 | 36.7±2.5 | 40.0±2.5 | 43.0±2.7 | 49.6±2.4‡⁎ | 50.6±2.2⁎ | 8.3 (0.2 to 16.4)‡ | 6.0 (−3.7 to 15.8) |
| N3, %TST | 33.2±2.7 | 28.0±2.3 | 33.6±2.4 | 24.4±2.5 | 17.6±2.1⁎ | 18.0±2.0⁎‡ | −1.5 (−10.3 to 7.3) | −6.7 (−13.4 to −0.1)‡ |
| REM, %TST | 22.5±2.4 | 29.7±1.9⁎ | 21.5±1.9 | 26.1±2.2 | 25.6±1.9 | 23.3±1.6 | −7.7 (−16.3 to 0.9) | −1.7 (−10.4 to 6.9) |
| NREM, min | 276±17 | 272±14 | 269±14 | 262±16 | 284±13 | 310±12⁎ | 26 (−37 to 89) | 56 (0 to 112) |
| Wake, min | 44±9 | 38±8 | 42±8 | 69±8 | 56±7 | 49±7⁎ | −7 (−34 to 21) | −18 (−50 to 14) |
| Awakenings, number | 22±3 | 17±2⁎ | 19±2 | 21±2 | 23±2‡ | 22±2 | 7 (1 to 14)‡ | 5 (−4 to 13) |
| Sleep Efficiency, % | 89±2 | 91±2 | 90±2 | 84±2 | 86±2 | 89±2⁎ | 1 (−6 to 7) | 5 (−3 to 13) |
| WASO, min | 27±5 | 19±4 | 17±4 | 33±5 | 24±4 | 24±3 | −1 (−24 to 22) | 1 (−22 to 25) |
| Micro-Arousal Index, 1/h | 8.0±1.4 | 6.9±1.1 | 7.1±1.2 | 7.1±1.2 | 10.1±1.0‡⁎ | 11.5±1.0‡⁎ | 4.1 (1.9 to 6.4)‡ | 5.4 (1.7 to 9.1)‡ |
| 24-hour ambulatory blood pressure | ||||||||
| 24 h BP | ||||||||
| Systolic BP, mmHg | 114±3 | 111±3⁎ | 115±3 | 117±3 | 119±3⁎‡ | 115±3 | 6 (3 to 8)‡ | −2 (−5 to 0) |
| Diastolic BP, mmHg | 74±2 | 71±2⁎ | 75±2 | 76±2 | 79±2⁎‡ | 77±2 | 7 (4 to 9)‡ | 1 (−1 to 3) |
| Mean BP, mmHg | 88±2 | 84±2⁎ | 88±2 | 89±2 | 92±2⁎‡ | 90±2 | 6 (4 to 8)‡ | 0 (−2 to 2) |
| Systolic-SD, mmHg | 13±1 | 12±1 | 13±1 | 13±1 | 13±1 | 13±1 | 1 (−2 to 3) | −1 (−3 to 2) |
| Diastolic-SD, mmHg | 12±1 | 10±1 | 11±1 | 11±1 | 11±1 | 11±1 | 1 (−1 to 3) | 0 (−2 to 2) |
| Heart rate, bpm | 72±2 | 70±2⁎ | 74±2⁎ | 74±2 | 79±2⁎‡ | 78±2⁎‡ | 8 (6 to 10)‡ | 3 (1 to 5)‡ |
| Day period1 | ||||||||
| Systolic BP, mmHg | 120±3 | 115±3⁎ | 119±3 | 124±3 | 122±3⁎‡ | 118±3⁎‡ | 3 (1 to 6)‡ | −4 (−7 to −2)‡ |
| Diastolic BP, mmHg | 79±2 | 75±2⁎ | 78±2 | 82±2 | 83±2‡ | 79±2⁎ | 6 (4 to 8)‡ | −1 (−3 to 1) |
| Mean BP, mmHg | 93±2 | 88±2⁎ | 92±2 | 96±2 | 96±2‡ | 92±2⁎‡ | 5 (3 to 7)‡ | −2 (−4 to 0)‡ |
| Systolic-SD, mmHg | 12±1 | 12±1 | 15±1⁎ | 12±1 | 13±1 | 14±1 | 2 (−2 to 5) | −1 (−4 to 3) |
| Diastolic-SD, mmHg | 11±1 | 10±1 | 12±1 | 11±1 | 12±1 | 11±1 | 2 (−1 to 5) | −1 (−4 to 2) |
| Heart rate, bpm | 74±2 | 72±2⁎ | 78±2⁎ | 77±2 | 85±2⁎‡ | 81±2⁎ | 9 (7 to 12)‡ | 1 (−2 to 3) |
| Night period1 | ||||||||
| Systolic BP, mmHg | 100±3 | 98±3 | 102±3 | 102±3 | 114±3⁎‡# | 104±3# | 14 (10 to 18)‡ | 1 (−4 to 5) |
| Diastolic BP, mmHg | 62±2 | 60±2⁎ | 64±2 | 63±2 | 71±2⁎‡# | 68±2⁎# | 11 (7 to 14)‡ | 3 (0 to 7) |
| Mean BP, mmHg | 75±2 | 72±2⁎ | 77±2 | 76±2 | 85±2⁎‡# | 80±2⁎# | 12 (9 to 15)‡ | 3 (−1 to 6) |
| Systolic-SD, mmHg | 13±1 | 12±1 | 12±1 | 14±1 | 13±1 | 13±1 | 0 (−4 to 3) | 0 (−4 to 3) |
| Diastolic-SD, mmHg | 12±1 | 10±1⁎ | 10±1⁎ | 12±1 | 11±1 | 11±1 | 1 (−2 to 4) | 1 (−2 to 4) |
| Heart rate, bpm | 67±2 | 62±2⁎ | 63±2⁎ | 66±2 | 68±2‡ | 68±2⁎‡# | 7 (4 to 11)‡ | 7 (3 to 11)‡ |
Values are presented as means ± SE and were obtained from multivariable regression analyses. All valid, single measurements obtained during the three 24-hours ambulatory blood pressure sessions were included in the regression analyses (a total of 4181 valid blood pressure measurements in 23 participants).
P<0.05 for difference in night-day change between groups. N1 to N3, sleep stage 1 to 3; TST, total sleep time; REM, rapid eye movement; NREM, non-rapid eye movement; WASO, wake after sleep onset; BP, blood pressure; SD, standard deviation.
Day- and night period was defined by the individual habit of each participant and was detected by the position sensor of the blood pressure device. Therefore, the night period started when a participant was in supine position for at least 2 consecutive blood pressure measurements. In the morning, the daytime period started when a participant was in standing or sitting position for at least 2 consecutive blood pressure measurements.
24-hour ambulatory blood pressure monitoring at baseline, during the 1st and during the 12th month at Dumont d'Urville (20 m, Panel A & B) or Concordia Station (3233 m, Panel C & D), respectively. Dots represent mean values of all measurements within the same hour of the day, separated for the time point. Whiskers represent the standard error of the mean of the corresponding hour of the day. Mean and SE values were calculated by linear mixed regression models using all valid, single blood pressure measurements available (4181 single measurements). *P<0.05 between diurnal/nocturnal blood pressure measurements obtained during the 1- (*) or 12- (*) month in Antarctica compared to baseline measurements. Panel E (DdU) and F (Concordia) represent the proportions of participants having normal (Dippers) or abnormal (Non-Dippers) day-to-night blood pressure reductions at the corresponding time points. *P<0.05 in the proportion of Dippers to Non-Dippers within the same group when staying in Antarctica compared to baseline (assessed by McNemar Tests of paired proportions).
This prospective cohort study confirms sleep disturbances and reveals elevated night BP in Antarctic overwintering crewmembers staying for 12 months at Concordia when compared to baseline and to a low-altitude control group. These alterations indicate persistent and uncompensated cardiovascular stress at Concordia for 1 year. The observed findings are probably driven by the difference in altitude and hypobaric hypoxia7 since confinement (e.g. psychological distress), climatic conditions (e.g. cold during outdoor activities) and altered daylight between Concordia and DdU were comparable, although this was not quantitatively assessed in this study. Our findings extend previous reports on altered cardiovascular function during acute (days) and prolonged (weeks) exposure to high altitude.4 Especially, nocturnal and not daytime BP remained elevated during 12 months, which is in accordance with the presence of more superficial sleep, hypoxia-related arousals and high-altitude periodic breathing.2,8 Moreover, a large proportion of crewmembers at Concordia showed insufficient day-to-night BP reductions, an important clinical marker for cardiovascular outcomes.5 These cardiovascular findings based on 4181 valid BP measurements are novel and of clinical importance since evacuation or treatment according to international guidelines in case of cardiovascular events is impossible at Concordia and in many Antarctic stations, but also in other remote high-altitude environments.
The limitations of this study are to provide no additional measurements during acute (days) Antarctic exposure; no gold-standard polysomnographies to assess sleep stages9 and additional cardiovascular risk factors, such as nocturnal arterial oxygenation or sleep-disordered breathing. However, the DREEM headband and algorithm have been validated against polysomnography6 and showed good reproducibility between the two consecutive nights in this study, with mean differences (95%CI) in sleep stages 2 and 3 (%TST) of −0.2%TST(−3.4 to 3.0) and 0.9%TST(−1.9 to 3.6). Due to the small sample size, it was not feasible to investigate explanatory factors behind the observed findings. However, a strength of this study was to prospectively follow for 12 months the Antarctic coastal DdU crewmembers. This control group accounts for many confounding factors in Antarctica and allows underlining the effect of chronic hypoxic exposure in Concordia.
Overall, our data emphasize persistent cardiovascular stress in the presence of impaired sleep and of hypoxia over a 12-month duration. Based on our findings, randomized clinical trials investigating long-term preventive measures, e.g. oxygen or acetazolamide therapy,10 against cardiovascular risk factors are warranted during long-term stays at Concordia.