The diaphragm is the main breathing muscle and contraction of the diaphragm is vital for ventilation so any disease that interferes with diaphragmatic innervation, contractile muscle function, or mechanical coupling to the chest wall can cause diaphragm dysfunction. Diaphragm dysfunction is associated with dyspnoea, intolerance to exercise, sleep disturbances, hypersomnia, with a potential impact on survival.
Diagnosis of diaphragm dysfunction is based on static and dynamic imaging tests (especially ultrasound) and pulmonary function and phrenic nerve stimulation tests. Treatment will depend on the symptoms and causes of the disease. The management of diaphragm dysfunction may include observation in asymptomatic patients with unilateral dysfunction, surgery (i.e., plication of the diaphragm), placement of a diaphragmatic pacemaker or invasive and/or non-invasive mechanical ventilation in symptomatic patients with bilateral paralysis of the diaphragm. This type of patient should be treated in experienced centres.
This review aims to provide an overview of the problem, with special emphasis on the diseases that cause diaphragmatic dysfunction and the diagnostic and therapeutic procedures most commonly employed in clinical practice. The ultimate goal is to establish a standard of care for diaphragmatic dysfunction.
The diaphragm separates the thoracic cavity from the abdominal cavity. It is the main breathing muscle and is innervated by the phrenic nerves (PN) that arise from nerve roots C3–C5. Diseases that interfere with diaphragmatic innervation, contractile muscle properties, or mechanical coupling to the chest wall can cause diaphragmatic dysfunction.1 Diaphragmatic dysfunction is associated with the presence of respiratory symptoms, especially dyspnoea, exercise intolerance, sleep disturbances, hypersomnia and, in the most severe cases, a negative impact on survival.2
The purpose of this review was to identify the causes of diaphragmatic dysfunction, determine signs of the disease, and set diagnostic criteria for the establishment of a standard of care.
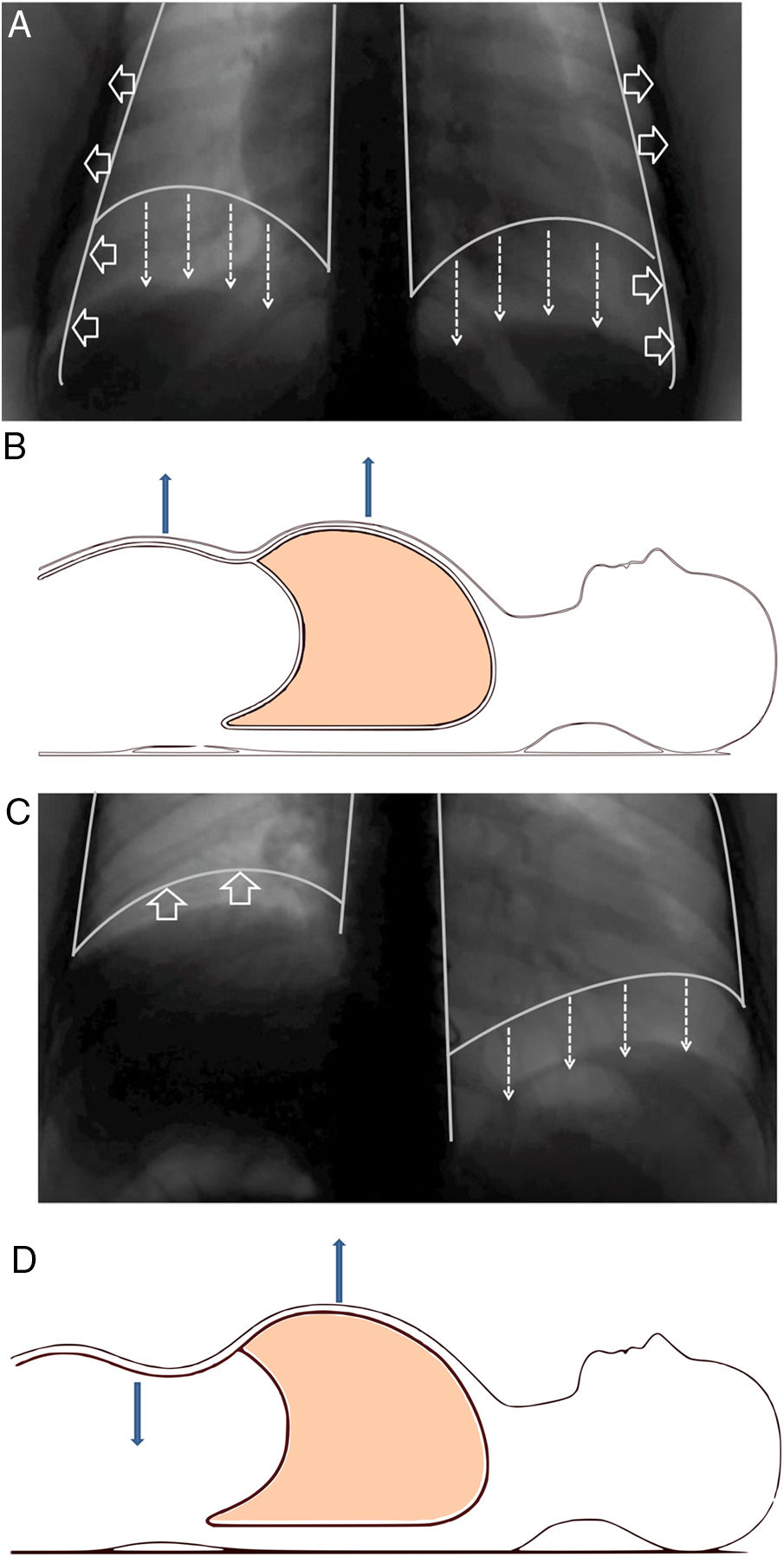
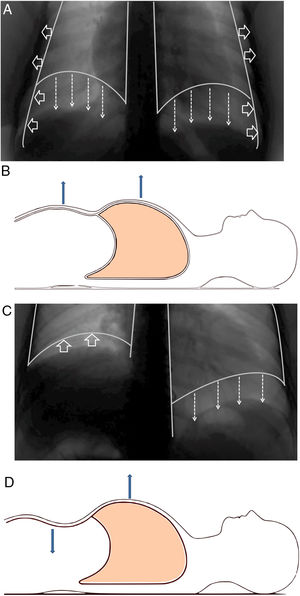
Structure and functionThe diaphragm is the main breathing muscle.2 During quiet inspiration, the dome shape of the diaphragm changes very little and the muscular action causes a shortening of the apposition zone (area in which the lower rib cage and the diaphragm are in direct contact) that makes the diaphragm to move caudally like a piston, thereby increasing abdominal pressure and decreasing pleural pressure. The latter is transmitted to the lung – causing it to insufflate – and the costal wall, which will tend to collapse. This action is compensated by an increase of abdominal pressure – which causes the thoracic cage to expand in the apposition area – and the contraction of the diaphragm in the lower ribs, which also opens the thoracic cage2–4 (Fig. 1A and B).
(A) Normal contraction of the diaphragm during quiet inspiration: the muscular action causes the diaphragm to move together like a piston in the caudal direction (direction of the arrows), thereby increasing abdominal pressure and decreasing pleural pressure. The latter is transmitted to the lung, causing it to be insufflated. (B) In supine position, it can be seen that both the rib cage and the abdomen move outwards. (C) When there is paralysis of the diaphragm (right side), the negative intrathoracic pressure drags the diaphragm and the abdominal viscera towards the thorax (direction of the arrows), which generates a negative abdominal pressure. (D) In supine position, it is observed how this negative abdominal pressure causes a paradoxical movement during inspiration: the abdomen moves inwards.
The term diaphragmatic dysfunction includes eventration, weakness and diaphragmatic paralysis.5 Eventration is a permanent elevation of all or part of the hemidiaphragm caused by thinning.5,6 Diaphragmatic weakness would be the partial loss of muscle strength to generate the necessary pressure for adequate ventilation,6,7 while paralysis means the total absence of this capacity. This disorder, depending on the cause, can be unilateral or bilateral, temporary or permanent.8 The hernia is the protrusion of an abdominal organ or tissue through a diaphragmatic defect. The most frequent congenital hernias are those of Bochdalek and Morgani6 and, of those acquired, the hiatus hernia.9 On chest X-ray they will be observed as a localized elevation of the diaphragm.
Another rare form of diaphragmatic dysfunction is diaphragmatic flutter. This dysfunction is characterized by the occurrence of repeated, variable-duration episodes of regular involuntary contractions. Signs of diaphragmatic flutter include pulsations in the epigastrium, dyspnoea and thoraco-abdominal pain. The aetiology of this condition is not well understood, and a standard of care has not been established yet. A set of trials have been performed with different agents, surgical ablation of the phrenic nerve and non-invasive ventilatory support, with varying results.10,11
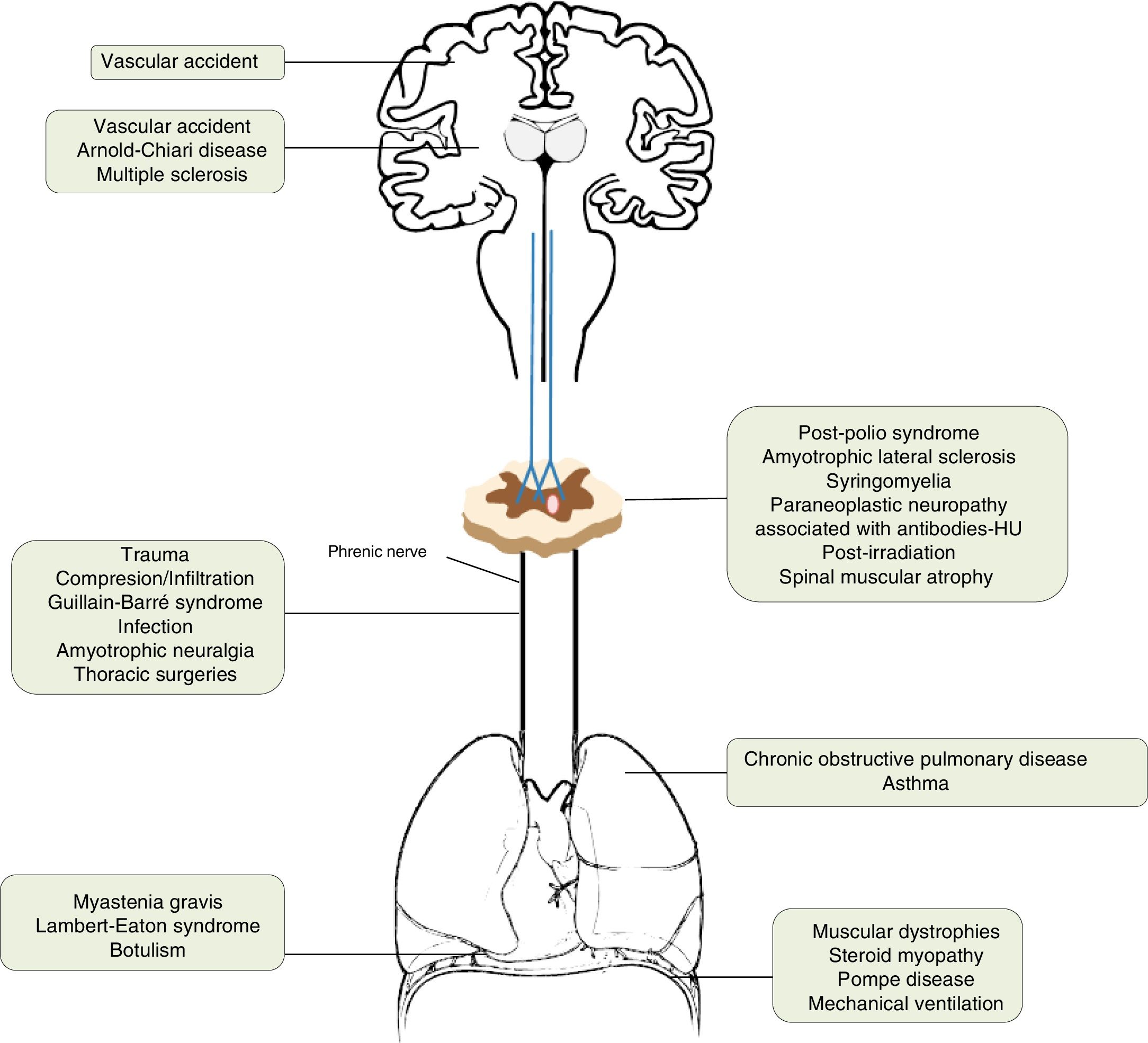
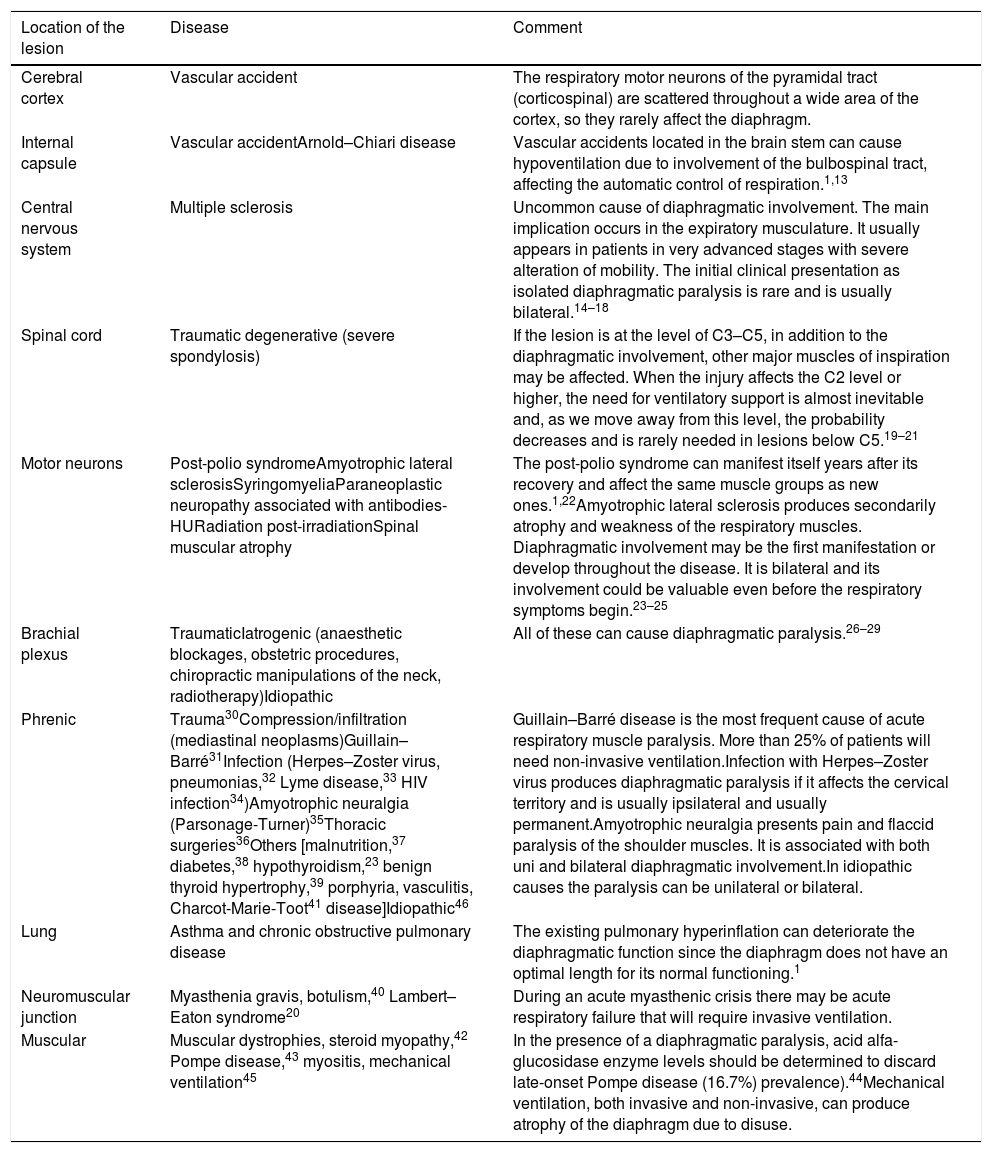
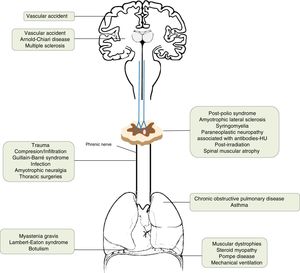
AetiologyThe incidence of diaphragmatic dysfunction is unknown, given the multiple diseases that cause it. The level of severity of this complication is determined either by the level of anatomical involvement or one-sidedness or bilaterality.2,7,12Fig. 2 shows by anatomical site the diseases that can cause diaphragmatic dysfunction from the cerebral cortex, through the internal capsule, the central nervous system, the spinal cord, the brachial plexus, the motor neurons and the PN, until reaching the neuromuscular synapse and the muscles themselves.1,13–46Table 1 shows its most relevant characteristics.
Causes that can cause diaphragmatic dysfunction.
| Location of the lesion | Disease | Comment |
|---|---|---|
| Cerebral cortex | Vascular accident | The respiratory motor neurons of the pyramidal tract (corticospinal) are scattered throughout a wide area of the cortex, so they rarely affect the diaphragm. |
| Internal capsule | Vascular accidentArnold–Chiari disease | Vascular accidents located in the brain stem can cause hypoventilation due to involvement of the bulbospinal tract, affecting the automatic control of respiration.1,13 |
| Central nervous system | Multiple sclerosis | Uncommon cause of diaphragmatic involvement. The main implication occurs in the expiratory musculature. It usually appears in patients in very advanced stages with severe alteration of mobility. The initial clinical presentation as isolated diaphragmatic paralysis is rare and is usually bilateral.14–18 |
| Spinal cord | Traumatic degenerative (severe spondylosis) | If the lesion is at the level of C3–C5, in addition to the diaphragmatic involvement, other major muscles of inspiration may be affected. When the injury affects the C2 level or higher, the need for ventilatory support is almost inevitable and, as we move away from this level, the probability decreases and is rarely needed in lesions below C5.19–21 |
| Motor neurons | Post-polio syndromeAmyotrophic lateral sclerosisSyringomyeliaParaneoplastic neuropathy associated with antibodies-HURadiation post-irradiationSpinal muscular atrophy | The post-polio syndrome can manifest itself years after its recovery and affect the same muscle groups as new ones.1,22Amyotrophic lateral sclerosis produces secondarily atrophy and weakness of the respiratory muscles. Diaphragmatic involvement may be the first manifestation or develop throughout the disease. It is bilateral and its involvement could be valuable even before the respiratory symptoms begin.23–25 |
| Brachial plexus | TraumaticIatrogenic (anaesthetic blockages, obstetric procedures, chiropractic manipulations of the neck, radiotherapy)Idiopathic | All of these can cause diaphragmatic paralysis.26–29 |
| Phrenic | Trauma30Compression/infiltration (mediastinal neoplasms)Guillain–Barré31Infection (Herpes–Zoster virus, pneumonias,32 Lyme disease,33 HIV infection34)Amyotrophic neuralgia (Parsonage-Turner)35Thoracic surgeries36Others [malnutrition,37 diabetes,38 hypothyroidism,23 benign thyroid hypertrophy,39 porphyria, vasculitis, Charcot-Marie-Toot41 disease]Idiopathic46 | Guillain–Barré disease is the most frequent cause of acute respiratory muscle paralysis. More than 25% of patients will need non-invasive ventilation.Infection with Herpes–Zoster virus produces diaphragmatic paralysis if it affects the cervical territory and is usually ipsilateral and usually permanent.Amyotrophic neuralgia presents pain and flaccid paralysis of the shoulder muscles. It is associated with both uni and bilateral diaphragmatic involvement.In idiopathic causes the paralysis can be unilateral or bilateral. |
| Lung | Asthma and chronic obstructive pulmonary disease | The existing pulmonary hyperinflation can deteriorate the diaphragmatic function since the diaphragm does not have an optimal length for its normal functioning.1 |
| Neuromuscular junction | Myasthenia gravis, botulism,40 Lambert–Eaton syndrome20 | During an acute myasthenic crisis there may be acute respiratory failure that will require invasive ventilation. |
| Muscular | Muscular dystrophies, steroid myopathy,42 Pompe disease,43 myositis, mechanical ventilation45 | In the presence of a diaphragmatic paralysis, acid alfa-glucosidase enzyme levels should be determined to discard late-onset Pompe disease (16.7%) prevalence).44Mechanical ventilation, both invasive and non-invasive, can produce atrophy of the diaphragm due to disuse. |
Alterations such as hypokalemia, hypophosphatemia, hypomagnesemia or metabolic alkalosis; some connective tissue diseases, such as Shrinking lung syndrome (rare presentation of systemic lupus erythematosus that presents with respiratory distress and restrictive functional impairment); percutaneous punctures of veins (subclavian and internal jugular); placement of intercostal drainages; radiofrequency ablation; or chronic sclerosing mediastinitis, may also co-occur with diaphragmatic dysfunction.2,7,42,47–52
Clinical presentationUnilateral diaphragmatic dysfunction may be asymptomatic,53 which explains why it is often diagnosed incidentally5 when an elevation is observed in a hemidiaphragm on chest X-ray performed for another reason. Symptoms are usually more severe in obese patients or patients with an associated cardiac or pulmonary pathology.2,7 The most frequent symptoms are dyspnoea on exertion and orthopnea,53,54 but there may also be symptoms of nocturnal hypoventilation and gastroesophageal reflux.55
Physical examination is non-specific: decreased respiratory sounds at the base of the affected hemithorax and possible dullness to percussion.56 Paradoxical thoraco-abdominal movement during sleep occurs occasionally. Some studies have revealed that these patients tend to sleep with the healthy hemidiaphragm in the lower part.57
When there is bilateral involvement, patients usually show symptoms of orthopnoea. Dyspnoea – which may occur at rest – becomes evident during immersion in water.58 Patients usually show cyanosis, bilateral diminution of breathing sounds, rapid and superficial respiration, or paradoxical movement of the abdominal wall,7,59 especially when the patient is in decubitus;2,60 this is due to the “passive” behaviour of the diaphragm during inspiration. When the diaphragm is paralyzed, inspiration is obtained thanks to the contraction of the external intercostal muscles and accessory muscles (sternocleidomastoids, scalenes), which will expand the rib cage and generate intrathoracic negative pressure. This pressure will “drag” the diaphragm and abdominal viscera towards the thorax, which will generate a negative abdominal pressure and, therefore, a decrease in the anterior abdominal wall60 (Fig. 1C and D). Most patients with diaphragmatic involvement have sleep disorders and significant hypoventilation, especially during REM sleep, with its related symptoms.61,62Table 2 shows the most relevant differences between unilateral or bilateral diaphragmatic paralysis.
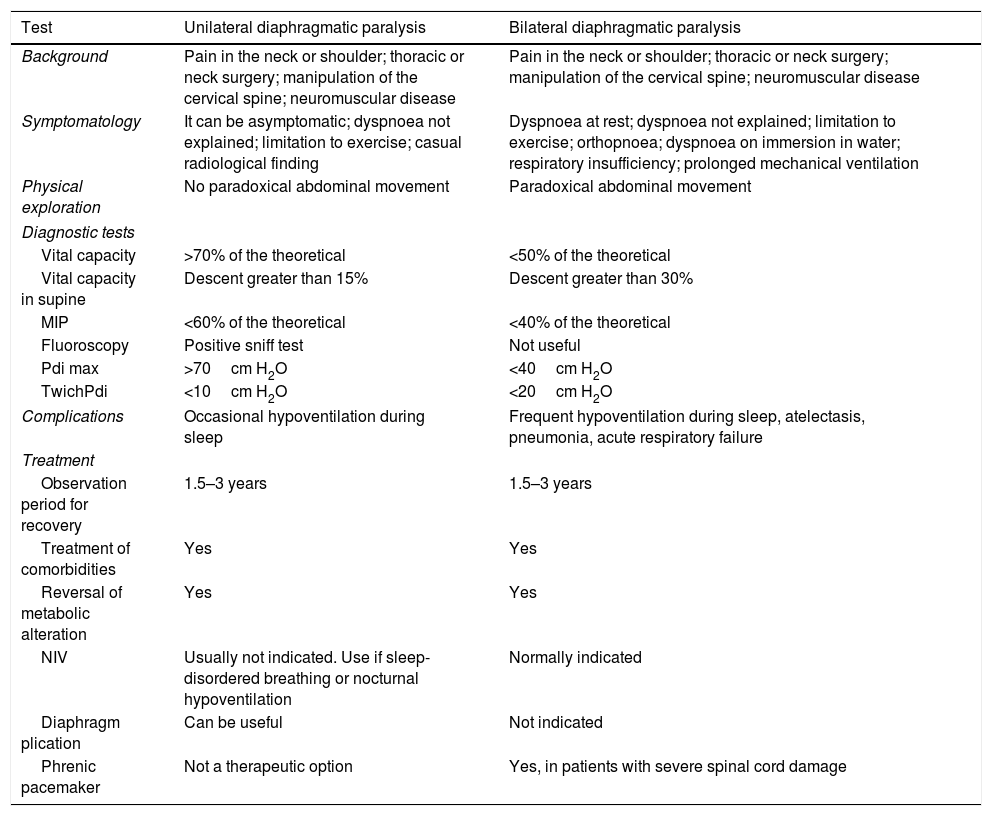
Comparison of the clinical history, diagnostic tests and treatments according to diaphragmatic paralysis, either unilateral or bilateral (modified by McCool and Tzelepis)7
| Test | Unilateral diaphragmatic paralysis | Bilateral diaphragmatic paralysis |
|---|---|---|
| Background | Pain in the neck or shoulder; thoracic or neck surgery; manipulation of the cervical spine; neuromuscular disease | Pain in the neck or shoulder; thoracic or neck surgery; manipulation of the cervical spine; neuromuscular disease |
| Symptomatology | It can be asymptomatic; dyspnoea not explained; limitation to exercise; casual radiological finding | Dyspnoea at rest; dyspnoea not explained; limitation to exercise; orthopnoea; dyspnoea on immersion in water; respiratory insufficiency; prolonged mechanical ventilation |
| Physical exploration | No paradoxical abdominal movement | Paradoxical abdominal movement |
| Diagnostic tests | ||
| Vital capacity | >70% of the theoretical | <50% of the theoretical |
| Vital capacity in supine | Descent greater than 15% | Descent greater than 30% |
| MIP | <60% of the theoretical | <40% of the theoretical |
| Fluoroscopy | Positive sniff test | Not useful |
| Pdi max | >70cm H2O | <40cm H2O |
| TwichPdi | <10cm H2O | <20cm H2O |
| Complications | Occasional hypoventilation during sleep | Frequent hypoventilation during sleep, atelectasis, pneumonia, acute respiratory failure |
| Treatment | ||
| Observation period for recovery | 1.5–3 years | 1.5–3 years |
| Treatment of comorbidities | Yes | Yes |
| Reversal of metabolic alteration | Yes | Yes |
| NIV | Usually not indicated. Use if sleep-disordered breathing or nocturnal hypoventilation | Normally indicated |
| Diaphragm plication | Can be useful | Not indicated |
| Phrenic pacemaker | Not a therapeutic option | Yes, in patients with severe spinal cord damage |
MIP, maximum inspiratory pressure; NIV, non-invasive ventilation; Pdi max, transdiaphragmatic pressure after maximum inspiratory effort with closed glottis; TwichPdi, transdiaphragmatic pressure after transcutaneous electrical or magnetic stimulation of the phrenic nerve.
Patients with unilateral diaphragmatic dysfunction usually exhibit respiratory sleep disorders (fatigue, daytime sleepiness, snoring and apnea). Thus, some authors recommend that all patients with eventration or diaphragmatic paralysis undergo a full-night polysomnography.63 Respiratory events generally include central hypopneas during REM sleep. These events often coincide with repeated episodes of desaturation that can be observed by pulse oximetry and are related to diaphragmatic weakness and paradoxical breathing. Desaturation is more frequent and severe when the patient is in lateral decubitus on the affected side.64
Patients with bilateral diaphragmatic dysfunction show the same symptoms and desaturation events, although they are more likely to experience orthopnea.62 The standard of treatment for patients with (unilateral or bilateral) diaphragmatic dysfunction and respiratory sleep disorders is continuous positive airway pressure or non-invasive mechanical ventilation. Yet, continuous positive airway pressure is more likely to fail in patients with bilateral diaphragmatic dysfunction, who will ultimately require non-invasive ventilation. Therefore, pressure titration should be performed in a sleep laboratory.65
There is a range of potential pathophysiological mechanisms of hypercapnic respiratory failure in obese patients. Some of these mechanisms include diaphragmatic dysfunction secondary to the accumulation of adipose tissue and related mechanical alterations (inappropriate length-tension relationship66). In the hypoventilation-obesity syndrome, the mechanisms that cause hypoventilation are complex and multifactorial. The role of diaphragmatic weakness in hypoventilation in obese patients is not well-understood; however, obesity seems to add an additional load to the respiratory system.67
DiagnosisSuspicion of diaphragmatic dysfunction may arise from the study of unexplained dyspnoea or, occasionally, after the casual finding of a diaphragmatic elevation in an imaging test performed for another purpose. Whatever it is, diagnosis is usually based on imaging tests – both static and dynamic – including radiography, fluoroscopy and chest ultrasound. Table 2 summarizes the most relevant diagnostic tests for unilateral and bilateral diaphragmatic paralysis.
RadiographyChest X-ray is a simple effective test to evaluate the pulmonary parenchyma in search of other potential causes of dyspnoea.12 X-ray allows physicians to see the structure, morphology and elevation of the diaphragm, has moderate interobserver reliability, and shows slightly more elevated values for the right hemidiaphragm.68 Its sensitivity, specificity, positive and negative predictive value for the diagnosis of unilateral diaphragmatic paralysis are 90%, 44%, 33% and 93%, respectively.68 However, in other studies, its sensitivity has not reached 70%.69 In bilateral diaphragmatic paralysis, the typical finding is the elevation of the two hemidiaphragms, which is associated with small pulmonary volumes and bibasal atelectasis.58 Although the presence of a diaphragmatic elevation is not necessarily a sign of dysfunction, its absence makes diaphragmatic dysfunction unlikely.68
FluoroscopyIt is a test that allows us to visualize the diaphragm continuously throughout the normal respiratory cycle and during the execution of forced inspiratory manoeuvres. It is an easy-to-use and -interpret technique5 with good inter-observer reliability70 and, for years, it has been the gold standard for the diagnosis of diaphragmatic paralysis.69 However, in some patients with bilateral diaphragmatic weakness, fluoroscopy findings can be misinterpreted, as some patients in the standing position may adopt an unusual respiratory pattern to compensate for their lack of mobility.2,58 This mechanism of compensation may be misinterpreted in the fluoroscopy as a diaphragmatic contraction.60 This situation can be prevented if the patient is in recumbent position, which is why some authors recommend that fluoroscopy is performed in this position. Therefore, fluoroscopy is a useful test for the diagnosis of unilateral hemidiaphragmatic paralysis. Conversely, fluoroscopy is not as useful for bilateral dysfunction, as findings can be misinterpreted. It should be performed with the patient in upright position (frontal and lateral) or in decubitus by an expert radiologist.
UltrasoundDiaphragmatic ultrasound is a non-invasive, portable, quick to perform, simple and well-tolerated test with a linear relationship between diaphragmatic movement and inspired volume, which allows quantitative and qualitative assessment of diaphragmatic movement.71 Thus, ultrasound been suggested as the technique of choice for assessing diaphragmatic movement on suspicion of malfunctioning.72 In addition, there is no exposure to ionizing radiation and intense patient cooperation is not essential.69 In expert hands, and following the appropriate methodology,73 it is a very reproducible technique, with good inter and intra-observer reliability and good reproducibility.71–74 The thickness of the diaphragm can be determined in more than 85% of measurements, with a low coefficient of variation (0.09–0.14).73 Variability of diaphragmatic movement can also be determined in virtually all measurements, with a good intra- and inter-observer correlation.75,76 Ultrasound has shown to be useful for the detection of diaphragmatic dysfunction,77 with a high sensitivity (93%) and specificity (100%) for diaphragmatic neuromuscular disease.78 At present, many authors consider ultrasound the method of choice for the evaluation of diaphragmatic movement.72,74,79
Hemidiaphragm visualization by ultrasound is achieved from an anterior approach, with the patient in supine position performing different breathing manoeuvres (quiet, deep breathing and sniff). Examination must start with the patient lying on the “healthy” side if unilateral paralysis is suspected.56,69,75
The thickening of the diaphragm (Tdi) indicates a shortening of the diaphragm. Its absence during inspiration confirms diaphragmatic paralysis. If there is muscle atrophy, thickness decreases and the diaphragm does not contract during inspiration.73,80 The lower limit of normal diaphragmatic thickness at rest (at the end of an unforced expiration) in most patients is 1.5mm.81 The two indexes usually used for the diagnosis of diaphragmatic paralysis include a Tdi value <2mm and a diaphragm thickening fraction (TFdi) value <20% [TFdi: (thickness at the end of the inspiration−thickness at the end of expiration)/thickness at the end of expiration (in %)].80 The normal lower limit accepted for the TFdi is 20%.
The normal movement of the diaphragm during inspiration is caudal, so the line corresponding to the diaphragm (echogenic line located between the liver or spleen and the lung) moves downward (approaching the transducer), preceded by a pause. Diaphragmatic paralysis shows an absence of caudal movement of the diaphragm during normal inspiration, or a paradoxical movement of the diaphragm during the sniff test and occasionally with deep inspiration.5,69,82 Diaphragmatic weakness is determined where there is decreased amplitude of movement during deep breathing – with or without paradoxical movement during the sniff manoeuvre.
Pulmonary function testsPulmonary function tests are relevant to the diagnosis of diaphragmatic dysfunction. In general, weakness of the inspiratory muscles usually leads to a restrictive pattern, with a decrease in the total pulmonary, vital and functional residual capacities, keeping the CO diffusion and the residual volume preserved. The FEV1/FVC ratio is also relatively preserved.54,57 The measurement of vital capacity is of great value. On the one hand, when vital capacity is normal, relevant inspiratory muscle weakness is unlikely.83 On the other hand, a more severe decrease of 15–30% when going from the sitting position to decubitus – depending on whether paralysis is unilateral or bilateral – suggests some degree of diaphragmatic weakness and requires further examination.2
One way of estimating the strength of respiratory muscles is by measuring the pressures they generate at different points in the airway. To do this, two types of tests can be used: (a) non-invasive tests, which determine the pressures generated in the mouth, nose or endotracheal tube;84 and (b) invasive tests, which require the placement of pressure probes in the stomach and/or oesophagus that will serve as a reflex of abdominal and pleural pressure, respectively.
Determination of maximum static pressures in the mouth during inspiration (MIP) and expiration (MEP) with the airway closed is considered a reasonable method for measuring the force generated jointly by the inspiratory and expiratory muscles. In addition, it is one of the most widely used techniques in clinical practice. This technique is easy to perform and well tolerated.85 Its greatest disadvantage is that it is highly dependent on the cooperation and effort of the patient.86 In general, absolute values of MIP above 80cm H2O in men and 70cm H2O in women exclude clinically relevant inspiratory muscle weakness.83 Normal MEP combined with low MIP suggests the existence of isolated weakness of the diaphragm.85 Finally, the concomitant reduction of MIP and MEP suggests that diaphragmatic involvement may be due to a generalized process, with simultaneous involvement of the inspiratory and expiratory muscles.7 In percentage values, MIP is around 60% of the predicted value (on average) in unilateral affectation87 vs. 40% in bilateral dysfunction.88 Nonetheless, a diminished MIP is not exclusive to muscular weakness and can be observed in patients with chronic obstructive pulmonary disease.89
The nasal sniff manoeuvre is used do determine inspiratory pressures in the nose and involves the performance of a rapid voluntary inspiratory effort through the nasal passages. It is a useful test for evaluating the strength of the diaphragm in clinical practice.85 A pressure, in absolute values, greater than 70mm Hg in men and 60mm Hg in women is unlikely to be associated with significant inspiratory muscle weakness.85
The most widely used invasive tests include oesophageal pressure (Pes) and transdiaphragmatic pressure (Pdi) measurement by estimating the difference between Pes (intrathoracic pressure) and gastric pressure (Pga) (intra-abdominal pressure) [Pdi=Pes−Pga]. Pes and Pdi can be obtained during maximum voluntary efforts, the most frequent being the sniff test (Sniff Pdi). Pdi is specific to diaphragm contraction and is the gold standard method for the evaluation of diaphragm function. Also, Pdi is the only reliable diagnostic method for bilateral paralysis.89 If an inspiratory effort is made with the paralyzed diaphragm, Pes and Pga will be negative and, therefore, the Pdi will not change.85 In clinical practice, Sniff Pes and Sniff Pdi are the two most reproducible voluntary tests for assessing overall respiratory and diaphragmatic force.83 A value of Sniff Pdi>100cm H2O in men and 80cm H2O in women make the existence of clinically significant diaphragmatic weakness unlikely.85 A Pdi of 0 confirms bilateral diaphragmatic paralysis89 although some authors have established the cut-off point at <10mm Hg.
Stimulation of the phrenic nerveThe gold standard method for the quantification of the mechanical function of the diaphragm is by measuring the negative pressure generated by its contraction in response to the stimulation of the PN.85 This method offers the possibility of activating and studying the diaphragm separately without the activation and concomitant action of other muscle groups. During stimulation, negative pressure can be monitored by calculating the difference between oesophageal and gastric pressures (Twitch Pdi). Transcutaneous electrical phrenic stimulation can be performed at the level of the neck unilaterally or bilaterally. However, this technique causes the patient discomfort and is technically more difficult in obese patients or in patients with anatomical alterations. The magnetic stimulation of PNs is usually applied bilaterally at the level of the cervical spine90; it is reproducible, easy to perform and well tolerated by patients. A Twitch Pdi<10–20cm H2O (depending on whether involvement is unilateral or bilateral) is generally suggestive of diaphragmatic dysfunction.91 Measuring Sniff Pdi and Twitch Pdi allows differential diagnosis of diaphragmatic paralysis caused either by first or second motor neuron involvement, a central cause, or lack of cooperation.92
Although electromyography and the stimulation test must be performed by experienced operators, they are very accurate in the assessment of neural and muscular disorders.
Electromyography is performed by the insertion of a needle electrode. This test can show abnormal spontaneous activity of the diaphragm, and it can also show different characteristics of motor unit potential, like amplitude, shape or recruitment.93 The uses of electromyography in the examination of respiratory muscles are described in specific guides.85
Findings in electromyography are supported by evidence obtained in other functional tests – such as PN conduction studies. Electromyography is a very useful method for determining the diagnosis, evolution and prognosis of PN disorders. Although electromyography is associated with potential complications, it has been demonstrated to be safe.94
Stimulation tests measure the efficacy of neural and neuromuscular transmission. They can be performed using electrical or magnetic stimulators. Electrical stimulators are less expensive and relatively selective but they cause the patients discomfort and the technique is complex. Magnetic stimulators are easy to use and cause less discomfort, but they are less selective and more expensive.
PN is stimulated at the level of the neck, and the electromyographic activity of the diaphragm is registered to measure PN latencies and amplitudes of muscle compound action potentials. In some neuromuscular disorders (i.e., demyelinating polyneuropathies), latencies are delayed due to slow PN conduction (6–8ms in healthy adults). In other settings (PN trauma) the amplitude of muscle action potentials can be decreased (normal amplitude values average 500–800mV). A lack of muscle action potential after phrenic stimulation is suggestive of diaphragmatic paralysis with a lesion near or at the neuromuscular junction. Cortical stimulation is usually performed using a magnetic stimulator to measure response time of the diaphragm. This time is compared with latency after direct stimulation of the PN, which yields central conduction time. Cortical stimulation is not selective and its application to the respiratory system is difficult.85
TreatmentThe treatment of diaphragmatic paralysis depends mainly on its cause and the symptomatology of the patient. In general, patients with asymptomatic unilateral involvement do not require treatment. Initially, all associated factors must be treated, including obesity, respiratory or chronic heart diseases, which could influence and increase the symptoms of paralysis. There are specific treatments when the aetiology of the paralysis is known and is potentially reversible, as in infectious processes,34,91 metabolic, endocrinological (such as diabetes94 or hypothyroidism95) or systemic erythematosus lupus (shrinking lung syndrome).47 We must also bear in mind that paralyzes of idiopathic cause – such as amyotrophic neuralgia – can resolve spontaneously.26,44 Other studies have shown that diaphragmatic paralysis of potentially reversible aetiology (surgical, paraneoplastic, diabetic neuropathy, etc.) can improve spontaneously the strength of the diaphragm and respiratory muscles by 40–60% of cases over time,96–98 suggesting the convenience of delaying any surgical approach.
During the observation period, the patient can be included in a specific respiratory rehabilitation plan.99 It has been shown that one-year inspiratory muscle training after cardiac surgery improves diaphragmatic mobility and the inspiratory muscle strength of patients with diaphragmatic dysfunction.100
Surgical diaphragmatic plicationThis is the main surgical correction treatment available to control dyspnoea in patients with diaphragmatic paralysis. It consists of folding the paralyzed diaphragm so that it is immobilized in a position of maximum inspiration, thereby relieving compression of the lung parenchyma and allowing lung reexpansion. It can be done through a thoracic (with thoracoscopy)99 or abdominal approach.101 It is primarily indicated for symptomatic patients with unilateral diaphragmatic dysfunction that – based on clinical, radiological and functional tests – has not resolved after a period of observation of 6–12 months and is therefore considered permanent and irreversible.99,102 Plication has also been successfully performed in some patients with bilateral involvement.102,103 In the series of patients operated on, the main causes of paralysis were traumatism, cardiac surgery and iatrogenic.99,104
Plication has been shown to be effective, safe and cause few complications,99,104–106 inducing an improvement of symptoms and dyspnoea.100,105 The beneficial effects of plication are not only visible on radiological scans101,104 but also in improved pulmonary function parameters.99,102,104,105 After surgery, improvements occur in the tidal volume of both hemidiaphragms (the operated and the healthy, probably related to a significant improvement in the expansion of the abdominal compartments of the rib cage),107 exercise capacity,108 daily activity and quality of life, with a reduction of up to 20 points in the score on Saint George's Respiratory Questionnaire.99,101,108 All this allows many patients to return to normal life. Morbid obesity, calcification of the diaphragm and certain neuromuscular diseases are relative contraindications.109
Phrenic nerve repair by microsurgeryThis surgical approach – which includes modalities such as local decompression, transposition or interposition of a nerve graft – can be indicated for patients with unilateral phrenic involvement of a mainly iatrogenic or traumatic origin who have not shown any clinical or radiological improvement in a reasonable period of time. It is necessary to previously demonstrate the continuity of the nerve and the viability of the neuromuscular plate through PN conduction studies and electromyography.110
Diaphragmatic pacemakerIt can be placed in patients with impaired bilateral mobility of the diaphragm who wish to delay the initiation of ventilation – both invasive and non-invasive – or who have started it but do not wish to continue or were not able to tolerate it. These patients generally exhibit cervical involvement at a level above C3, or with central alterations different from cervical involvement, – mainly congenital or acquired central hypoventilation. It can also be seen in patients with lower motor neuron involvement for a reason other than amyotrophic lateral sclerosis111 and in traumatological or idiopathic etiologies.112
The most relevant studies published so far on the use of a diaphragmatic pacemaker in amyotrophic lateral sclerosis have not confirmed its expected benefits, with higher mortality rates in patients using a pacemaker. Therefore, at present, it is not indicated for this type of patients.113,114 The patients to whom this treatment is offered must be strictly selected and studied in institutions with experience; the presence of severe nocturnal hypoventilation must be confirmed and PN, diaphragm, and lung function must be shown to be ideal.111
Ventilatory supportIt has been used successfully both in patients with unilateral and bilateral diaphragmatic paralysis, either permanently in the latter,115 or temporally in the former, until complete recovery of diaphragmatic function. Ventilatory support can be applied by invasive mechanical ventilation or non-invasive positive pressure ventilation (NPPV). NPPV is actually considered the tool of choice mainly in symptomatic patients with bilateral diaphragmatic paralysis. Tolerance is good,116 and it has been shown to provide both clinical and blood gas improvement in the long term.117 The indication of non-invasive ventilation would be similar to that for other neuromuscular or restrictive pathologies.118,119
Patients with acute respiratory failure may need intubation and mechanical ventilation, which can continue over time as a result of respiratory muscle paralysis. A study in 152 patients with spinal cord injury (50% with affectation at C3–C5 level) revealed that early tracheostomy reduces the duration of invasive ventilation and length of stay in the ICU; in addition, it decreases the incidence of complications associated with orotracheal intubation, except for ventilation-associated pneumonia.120 However, non invasive ventilation has been proposed as a weaning method prior to tracheostomy in collaborative patients with bilateral diaphragmatic paralysis, a small volume of secretions and an appropriate inspiratory flow.115
Tracheostomy and invasive ventilation can also be required by patients with neuromuscular disease when non-invasive ventilation has failed or invasive interventions are ineffective.121
Non-invasive ventilation is associated with some complications. Mild or transient complications are related to the use of masks. Severe complications can be caused by: (1) ventilation failure, which can be minimized by the strict selection of patients and the appropriate control of ventilation; (2) ventilation-associated pneumonia, with a lower risk in patients on invasive ventilation; (3) barotraumas, with a lower incidence than in patients on invasive ventilation; and (4) hypotension.122
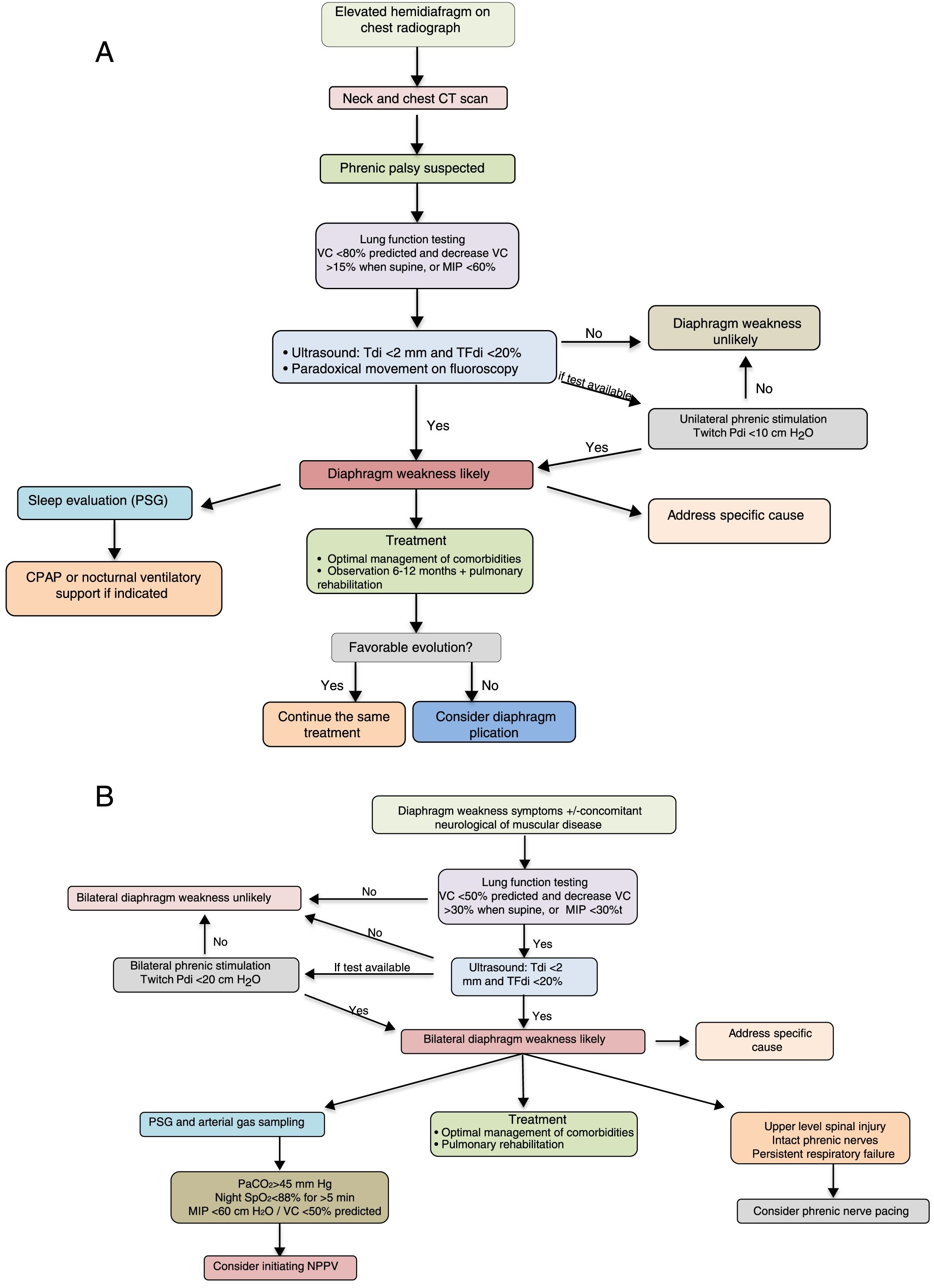
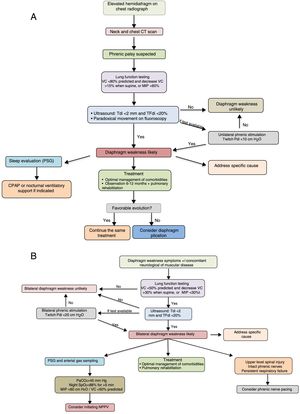
The diagnostic and therapeutic algorithms for unilateral and bilateral diaphragmatic paralysis are shown in Fig. 3A and B, respectively.
Suggestions for diagnostic and therapeutic algorithms in unilateral (A) and bilateral (B) diaphragmatic paralysis (modified from Dubé and Dres12). CPAP, continuous positive pressure in the airway; CT, computed tomography; GSA, arterial blood gases; MIP, maximum inspiratory pressure; NIV, non-invasive ventilation; Pdi, transdiaphragmatic pressure; NPSG, nocturnal polysomnography; SaO2, arterial oxygen saturation; TFdi, fraction of thickening of the diaphragm; VC, vital capacity.
In summary, diaphragmatic dysfunction can be associated with important clinical consequences. Identifying its origin and treating its symptoms and effects on sleep structure and exercise capacity requires thorough examination. Ultrasound is a simple and effective means of routinely assessing diaphragm function which guides clinicians in their therapeutic choice. Diaphragmatic dysfunctions should be treated in experienced centres, with access to diaphragmatic ultrasonography, phrenic stimulation, pacemaker placement, and surgical experience in diaphragmatic plication.
Author's contributionJorge Ricoy. Author. Conception and design. Writing of the article presented. Approval of the final version.
Nuria Rodríguez-Núñez. Co-author. Review of the submitted article. Approval of the final version.
José Manuel Álvarez-Dobaño. Co-author. Review of the submitted article. Approval of the final version.
María E. Toubes. Co-author. Review of the submitted article. Approval of the final version.
Vanessa Riveiro. Co-author. Review of the submitted article. Approval of the final version.
Luis Valdés. Author. Responsible. Conception and design. Writing of the article presented. Approval of the final version.
FundingThe authors declare the non-existence of external financing of this article.
Conflict of interestsWe wish to confirm that are no known conflicts of interest associated with this publication and there has been no financial support for this work that could have influenced its outcome.
The authors would like to thank José Ángel Novo-Platas for his help with the figures.