This scoping review summarized the evidence regarding the impact of acute exacerbations of COPD (AECOPD) on patients' health status beyond pulmonary function.
PubMed, Embase, and Web of Science were searched. Prospective cohort studies assessing the health status of patients with COPD in a stable phase of the disease and after a follow-up period (where at least one AECOPD occurred) were included. An integrated assessment framework of health status (i.e., physiological functioning, complaints, functional impairment, quality of life) was used.
Twenty-two studies were included. AECOPD acutely affected exercise tolerance, quadriceps muscle strength, physical activity levels, symptoms of dyspnoea and fatigue, and impact of the disease. Long-term effects on quadriceps muscle strength, symptoms of dyspnoea and depression, and quality of life were found. Repeated exacerbations negatively impacted the fat-free mass, levels of dyspnoea, impact of the disease and quality of life. Conflicting evidence was found regarding the impact of repeated exacerbations on exercise tolerance and physical activity levels.
AECOPD have well-established acute and long-term adverse effects on health status beyond pulmonary function; nevertheless, the recovery trajectory and the impact of repeated exacerbations are still poorly studied. Further prospective research is recommended to draw firm conclusions on these aspects.
Chronic obstructive pulmonary disease (COPD) is characterized by the onset of acute exacerbations (AECOPD), defined as an acute worsening of respiratory symptoms that result in additional therapy.1 On average, patients with COPD experience 1 to 4 AECOPD per year,2,3 which account for 50–70% of all COPD related-costs and increase their susceptibility to new AECOPD, hospitalization, and death.1,4,5 These events are associated with increased dyspnoea that usually lasts for 7 to 10 days, although in some cases there is no full recovery after weeks or months.1,6 Even a single AECOPD results in accelerated lung function decline and disease progression.7-9 Evidence also suggests that AECOPD lead to declines in exercise performance, functional status and quality of life (QoL), thus harming health status beyond pulmonary function.1,10-12 In addition, AECOPD are not random events but cluster together in time with a high-risk period for recurrent AECOPD in the 8-weeks following an initial exacerbation.1,13 Some patients are particularly susceptible to these (repeated) exacerbations and are known as the frequent exacerbator phenotype, which can be found across all disease severity groups.1,11 These frequent exacerbators seem to suffer from even more significant declines in lung function and QoL, potentially experiencing a further negative impact on health status.1,11,14-16
Health status can be defined as the impact of health on a person's ability to perform and derive fulfilment from daily life activities.17 Given its complexity, an integrated assessment framework of health status in COPD has been developed, which encompasses four sub-domains: physiological functioning, complaints, functional impairment and QoL.18 These sub-domains are relatively independent, and therefore a comprehensive assessment is essential to understand the impact of AECOPD on all health status domains and tailor interventions to counteract the detrimental effects that each person experiences.16,18-20 However, to date there are no reviews of the available evidence on the impact of AECOPD on the different health status domains. A scoping review to outline the existing evidence on this topic would provide the basis for future research to guide clinical practice on this matter.
Thus, this scoping review aimed to summarize and critically appraise the existing scientific evidence of the impact of AECOPD on the different sub-domains of health status in patients with COPD. Accordingly, our review question was: what do we know about the impact of AECOPD on the different sub-domains of health status in patients with COPD?
MethodsThis review followed the updated methodological framework to conduct scoping reviews proposed by Peters and colleagues,21 and is reported according to the Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews (PRISMA-ScR) checklist guidelines.22
Database and search strategyOne researcher (AM) performed an electronic literature search on PubMed, Embase and Web of Science from inception until January 2021. The following search strategy was used: ((COPD [title/abstract] OR chronic obstructive pulmonary disease [title/abstract/MeSH]) AND (hospital*[title] OR exacerbation [title/abstract])). The search results were imported to EndNote X9 (Clarivate Analytics, Philadelphia, PA, United States of America) and the duplicates were identified and removed.
A single researcher (AM or MSBG or CB) performed the title screening conservatively, i.e., excluding studies which clearly did not fulfil the criteria. Abstract and consequent full-text screening were performed independently by two out of three researchers (AM and CB or MSBG and CB). A consensus-based decision was made after discussion when discrepancies were present.
Selection criteriaStudies were included if they (i) studied patients with COPD that suffered from at least one AECOPD throughout the study; (ii) were prospective cohort studies; (iii) performed at least one type of health status assessment; and (iv) were written in English. Patients had to be assessed at baseline (in a stable phase of the disease) and after a follow-up period. The follow-up assessment(s) could have been conducted immediately after an AECOPD, during the course of a single AECOPD, or over a more extended period of time with the onset of AECOPD during the follow-up period being recorded (e.g., to compare changes in health status in frequent exacerbators vs. non-frequent exacerbators). Given the variability of used definitions of AECOPD in the literature, for the purpose of this review, AECOPD could be defined using symptom-based (i.e., patient-reported worsening of respiratory symptoms either to a healthcare professional or using a diary or tool) or event-based (i.e., change in treatment – medication and/or hospitalization) definitions, or a combination of both.23
Studies reporting on the short-, mid- or long-term effects of any intervention were excluded, unless it was possible to retrieve the data of a control group receiving only standard of care. Abstracts in conference proceedings were also excluded.
Based on the sub-classification of health status previously proposed for patients with COPD,18 we included measures of physiological functioning (exercise tolerance, muscle function and body composition), complaints (subjective complaints, expected dyspnoea and dyspnoea emotions), functional impairment (subjective impairment, behavioural impairment and actual physical activity) and QoL (general QoL, health-related QoL, satisfaction and relations). Measures of pulmonary function were not included.
Data extractionA customized data collection tool in Microsoft® Excel (Microsoft, Redmond, Washington, United States of America) and a data extraction table in Microsoft® Word (Microsoft, Redmond, Washington, United States of America) were developed to extract the most relevant information from the included studies and facilitate their subsequent analysis and interpretation. Data extraction was performed by AM and MSBG. Information on study design and timing of assessment, sample size, baseline characteristics (age, gender, body mass index (BMI), forced expiratory volume in one second (FEV1)), definition of (frequent) exacerbations used, AECOPD management and setting, measures of health status and main results regarding health status was collected.
Data synthesisData are presented in a tabularized format with a narrative summary linking the review results with the aim and review question. Key findings were categorized according to the sub-domains of health status proposed by Vercoulen and colleagues.18 In addition, whenever possible, results synthesis were grouped on acute vs. long-term effects of AECOPD, and on single vs. repeated AECOPD. For the purposes of this review, acute effects combined the results found regarding the onset and first days of an AECOPD, long-term effects combined the data on recovery/sustained changes over time, i.e., data from post-AECOPD periods, annual changes and follow-up times. Numerical summaries for the definition of (frequent) exacerbations, AECOPD characteristics and management, and measures of health status were collated. Schematic overviews were further developed to provide a visual synthesis of the results.
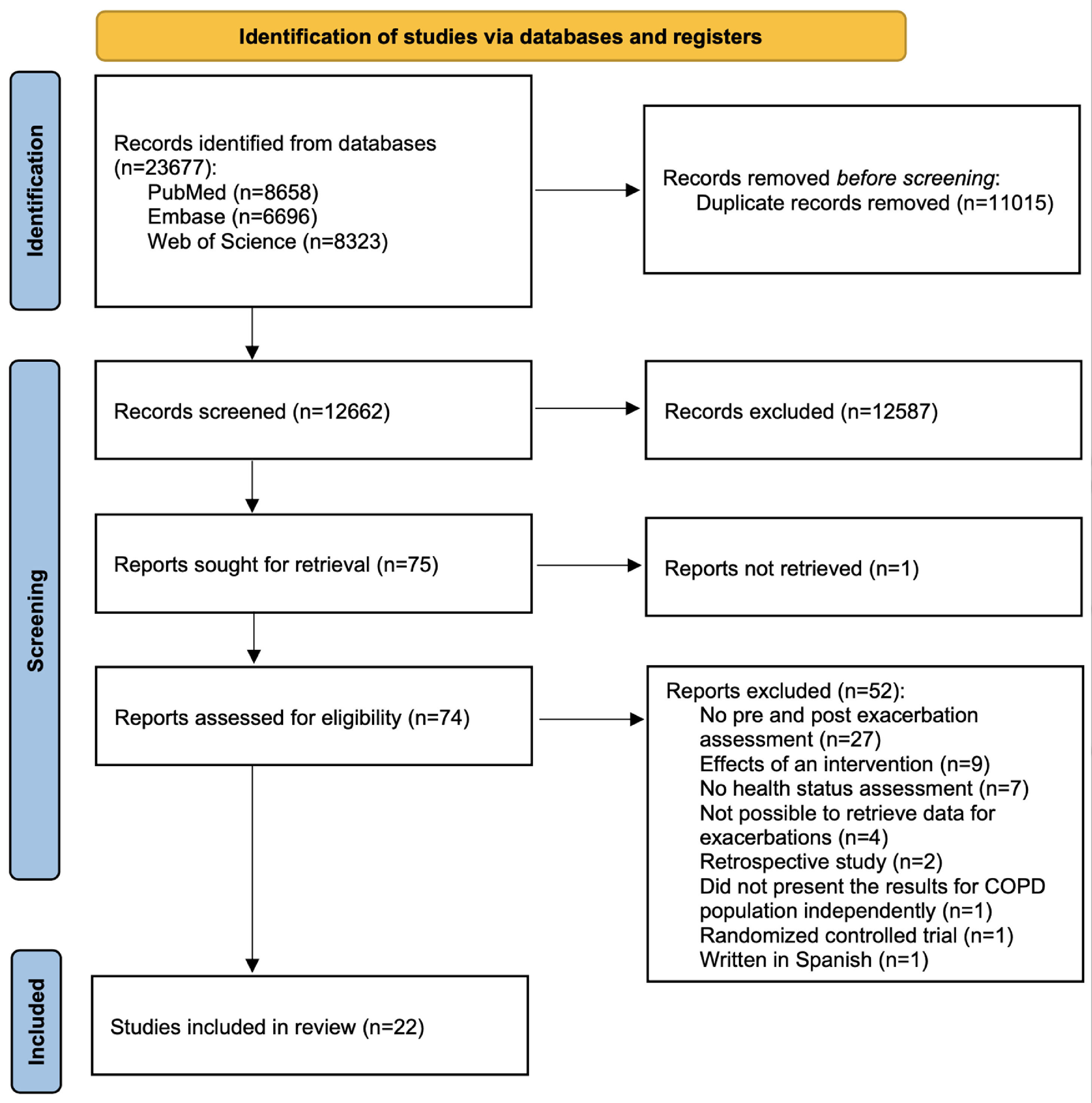
ResultsSearch resultsThe literature search provided a total of 23,677 records. After the removal of duplicates, 12,662 records were screened for relevant content using titles and abstracts. From these, 12,199 were excluded through title screening and 388 through abstract screening. Thus, the full text of 74 potentially relevant reports was assessed. Twenty-two studies were included in the review. The screening process is visualized in a flow chart (Fig. 1).
General characteristicsCharacteristics of the included studies are shown in Table 1. Studies were conducted between 2004 and 2020.
Influence of exacerbations on changes in health status over time in patients with COPD (n=22).
Data are presented as mean±standard deviation, median [quartile 1; quartile 3] or mean (standard error), unless otherwise stated.
6MWD, 6-min walk distance; 95%CI, 95% confidence interval; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; CAT, COPD assessment test; CCQ, Clinical COPD questionnaire; COPD, chronic obstructive pulmonary disease; CRQ, chronic respiratory disease questionnaire; EQ-5D, EuroQoL 5-dimension questionnaire; EXACT, exacerbations of chronic pulmonary disease tool; FEV1, forced expiratory volume in 1 s; GOLD, global initiative for chronic obstructive lung disease; MCID, minimal clinically important difference; mMRC, modified Medical Research Council dyspnoea questionnaire; OR, odds ratio; PAL, physical activity level; QMVC, quadriceps maximum voluntary contraction; SF-12, 12-item short form health survey; SF-36, 36-item short form health survey; SGRQ, Saint George's respiratory questionnaire; VMU, vector magnitude units.
Most studies (n = 14) defined AECOPD based on an increase in respiratory symptoms24-37 leading to changes in medication (n = 12) (e.g., treatment with antibiotics or systemic steroids)26,28,30,31,33-35,37-41 or to hospitalization (n = 4).35,38,39,41 Follow-up time varied from six weeks up to eight years. Ninety-one percent (n = 20) of the studies25,26,28-45 involved a sample combining patients that suffered a single exacerbation and patients that suffered repeated exacerbations. Severity of exacerbations was usually not reported. Nevertheless, 80% of the studies reporting this information were focused on moderate to severe AECOPD.24,26,28,29,37,40,41,45 Most reported treatment was composed by antibiotics, oral steroids or a combination of both (n = 10).25,26,28,29,34,37-41 Twelve studies included a sample combining patients treated at home with patients treated in the hospital.26-29,33,36-42
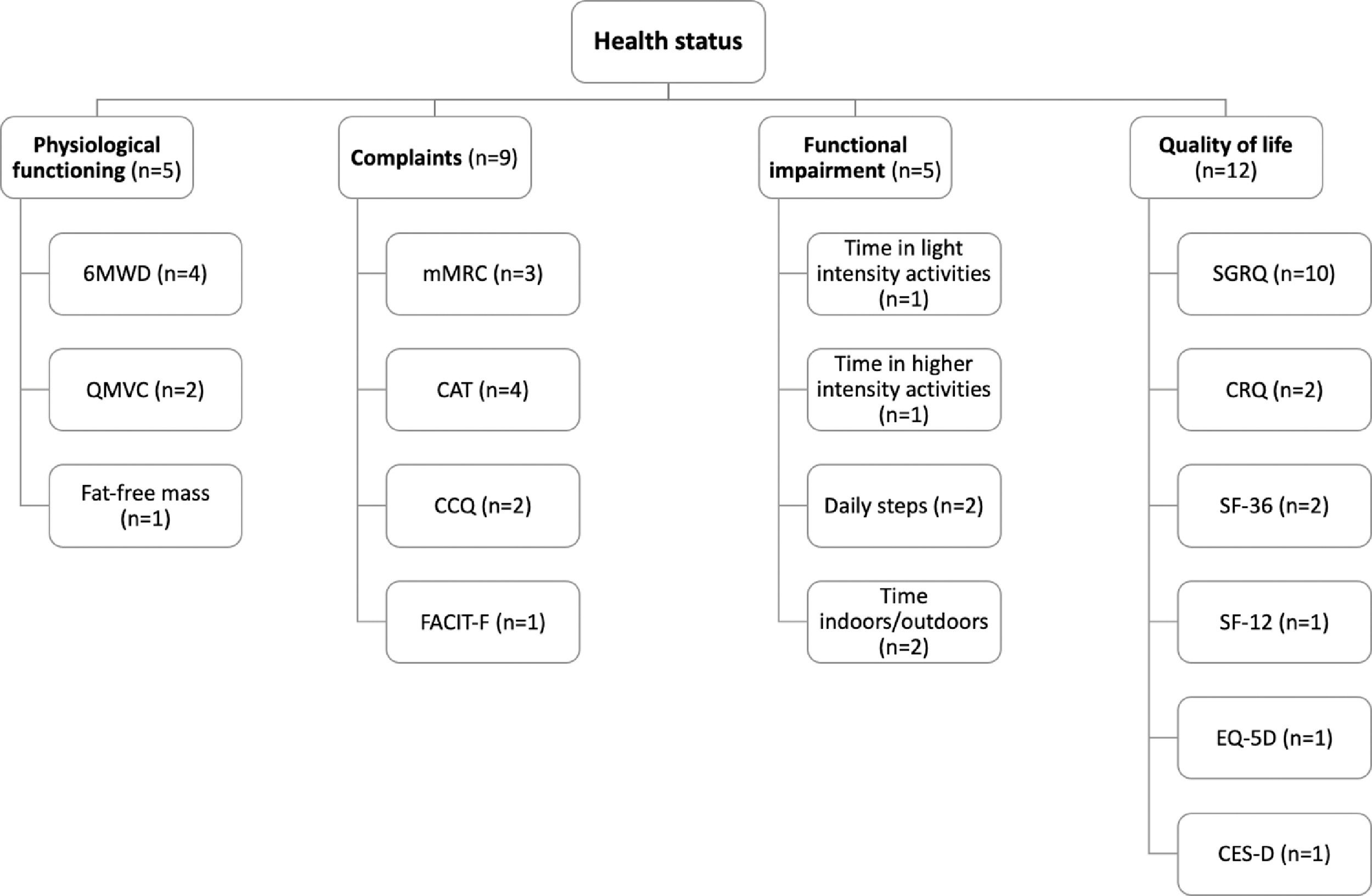
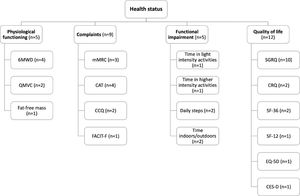
Tools used to assess health statusPhysiological functioning was assessed in five studies with the six-minute walking distance (6MWD),24,26,28,29 the quadriceps maximum voluntary contraction (QMVC)24,30 and the fat-free mass (FFM).30
Complaints were assessed in nine studies with the modified Medical Research Council dyspnoea questionnaire (mMRC),26,28,44 the COPD assessment test (CAT),35,37-39 the clinical COPD questionnaire (CCQ)35,36 and the functional assessment of chronic illness therapy-fatigue (FACIT-F).24
Functional impairment was assessed in five studies via physical activity levels.24,25,27,40,42 Physical activity was objectively assessed by daily step count25,40 and the time spent in light24 or higher intensity (i.e., >3000 vector magnitude units (VMU))42 activities. The time spent indoors/outdoors25,27 was quantified based on a diary.
Finally, QoL was assessed in twelve studies with the Saint George's respiratory questionnaire (SGRQ),27-29,31-34,43-45 the chronic respiratory disease questionnaire (CRQ),28,34 the 36-item short form health survey (SF-36),35,43 the 12-item short form health survey (SF-12),33 the EuroQoL 5-dimension questionnaire (EQ-5D)35 and the centre for epidemiologic studies depression scale (CES-D).41
Fig. 2 provides an overview of the outcome measures used to assess each health status subdomain. No study assessed all the domains that compose health status. Instead, the majority of studies25,30-34,36-43,45 (n = 15) focused on a single health status domain.
Schematic overview of the outcome measures used to assess each health status subdomain. *, activities >3000 vector magnitude units; 6MWD, six-minute walking distance; CAT, COPD assessment test; CCQ, clinical COPD questionnaire; CES-D, centre for epidemiological studies depression scale; CRQ, chronic respiratory disease questionnaire; EQ-5D, EuroQoL 5-dimension questionnaire; FACIT-F, functional assessment of chronic illness therapy-fatigue; mMRC, modified Medical Research Council dyspnoea questionnaire; QMVC, quadriceps maximum voluntary contraction; SF-12, 12-item short form health survey; SF-36, 36-item short form health survey; SGRQ, Saint George's respiratory questionnaire.
Two studies24,26 reported a significant reduction in the 6MWD in the first two to three days after the onset of an AECOPD, which was found to be more pronounced in patients with severe to very severe disease (GOLD stages 3 and 4).24 In addition, studies exploring the effects of repeated AECOPD26,28,29 also found a consistent decline in the 6MWD however, the recovery trajectory was conflicting. One study24 reported that the 6MWD increased back to the pre-exacerbation status after seven days, whereas more extended studies26,28 found that the observed decrease in 6MWD was maintained up to two years of follow-up, with inconsistent results regarding a lower 6MWD in frequent exacerbators (i.e., two or more AECOPD per year).26,28
QMVC was significantly reduced three and seven days after the onset of AECOPD symptoms.24 A decline in QMVC over a 1-year follow-up was also observed but with no correlation with having frequent exacerbations (i.e., two or more per year).30 Yet, frequent exacerbators had a more pronounced decline in FFM.30
Influence of exacerbations on complaintsA consistent worsening of complaints at the onset of an AECOPD was found across all outcome measures.24,26,36,37
There was an increase (worsening) in the mMRC score within 48 h of the onset of an AECOPD, which was more pronounced in patients suffering from repeated exacerbations.26 Furthermore, this increase was sustained at 1- and 2-years follow-up in frequent exacerbators (i.e., two or more AECOPD per year), but not in single exacerbators.26 A second study also corroborated these findings by reporting higher mMRC scores in frequent exacerbators.28
Similarly, a significant increase (worsening) in the CAT total score was found at the onset of an AECOPD, which was already noticeable one day before and was sustained for up to seven days after the onset of the exacerbation.37 Consistently, a faster deterioration in the CAT score (yearly change) was found in patients with exacerbations in comparison to non-exacerbators.35 In addition, patients presenting a sustained worsening on CAT score (i.e., increase ≥2 points) had a higher number of exacerbations.38,39
An increase (worsening) in the CCQ total score was found at the onset of an AECOPD, which recovered in the post-AECOPD assessment.36 This worsening was consistent across all CCQ domains.36 The CCQ score also deteriorated over time but without differences between exacerbators and non-exacerbators.35
A significant reduction (worsening) of 5 points in the FACIT-F score was observed at the onset of an AECOPD, which recovered to a 2 points reduction at day 3 in comparison to stable phase.24
Influence of exacerbations on functional impairmentDaily step count was reduced during the first 7 days of an AECOPD compared to a stable week.25 Further analysis of these data showed that it took a median of 3.5 days return to baseline levels, and patients with the largest falls in daily step count during the exacerbation were the ones taking longer to recover.25 Conflicting results were found regarding the annual decline, with one study25 finding a significantly faster decline in frequent exacerbators (i.e., two or more AECOPD per year) in comparison to infrequent exacerbators, and another study40 finding no differences between the decline of steps in frequent and infrequent exacerbators.
Time spent in activities >3000 VMU was reduced during AECOPD in comparison to the preceding or subsequent weeks.42 This decline was significant during the first week of exacerbation, with no further decline on the second week, and tended to increase back to baseline levels in the two subsequent weeks.42 In line with these results, time spent in light activities was higher during the first week post-exacerbation than the second week, with frequent exacerbators (i.e., two or more AECOPD in the preceding year) presenting a more considerable reduction in time spent in light activities from week 1 to week 2 in comparison to infrequent exacerbators.24
There was an increase in time spent indoors during exacerbations, which was sustained in the post-exacerbation period (days 1 to 35 after the onset) in comparison to the stable phase.27 A decrease in outdoors time during AECOPD that continues in the post-exacerbation period was also found, with frequent exacerbators (i.e., at least 2.5 AECOPD per year) presenting a faster annual decline in daily time outdoors than infrequent exacerbators.27 However, inconsistent results were found as in another study,25 in patients with a similar severity of AECOPD but better baseline lung function, the fall in time and percentage of days outdoors during exacerbations did not reach statistical significance.
Influence of exacerbations on quality of lifeA consistent increase (worsening) in the SGRQ score with exacerbations27,29,34,43,45 was reported, with frequent exacerbators presenting faster and more pronounced declines in QoL.28,29,31,33,34,43-45 In a study with a 2-year follow-up, patients who suffered a single AECOPD improved in SGRQ score by −3.8 points while frequent exacerbators (i.e., two or more AECOPD per year) worsened +2.4 points.32
A similar pattern for QoL has also been found in the CRQ, SF-36 and SF-12. Patients with AECOPD presented a decline in CRQ, SF-36 and SF-12 scores, which was more pronounced in frequent exacerbators across all CRQ domains and in the physical component of SF-36 and SF-12.28,33-35,43 The EQ-5D was found to decline annually in exacerbators and non-exacerbators, similarly.35
A strong relationship between the number of AECOPD during the first year of follow-up and the change in depression score (i.e., CES-D) over 3-years has been found, with patients who suffered from more exacerbations presenting the most significant declines.41
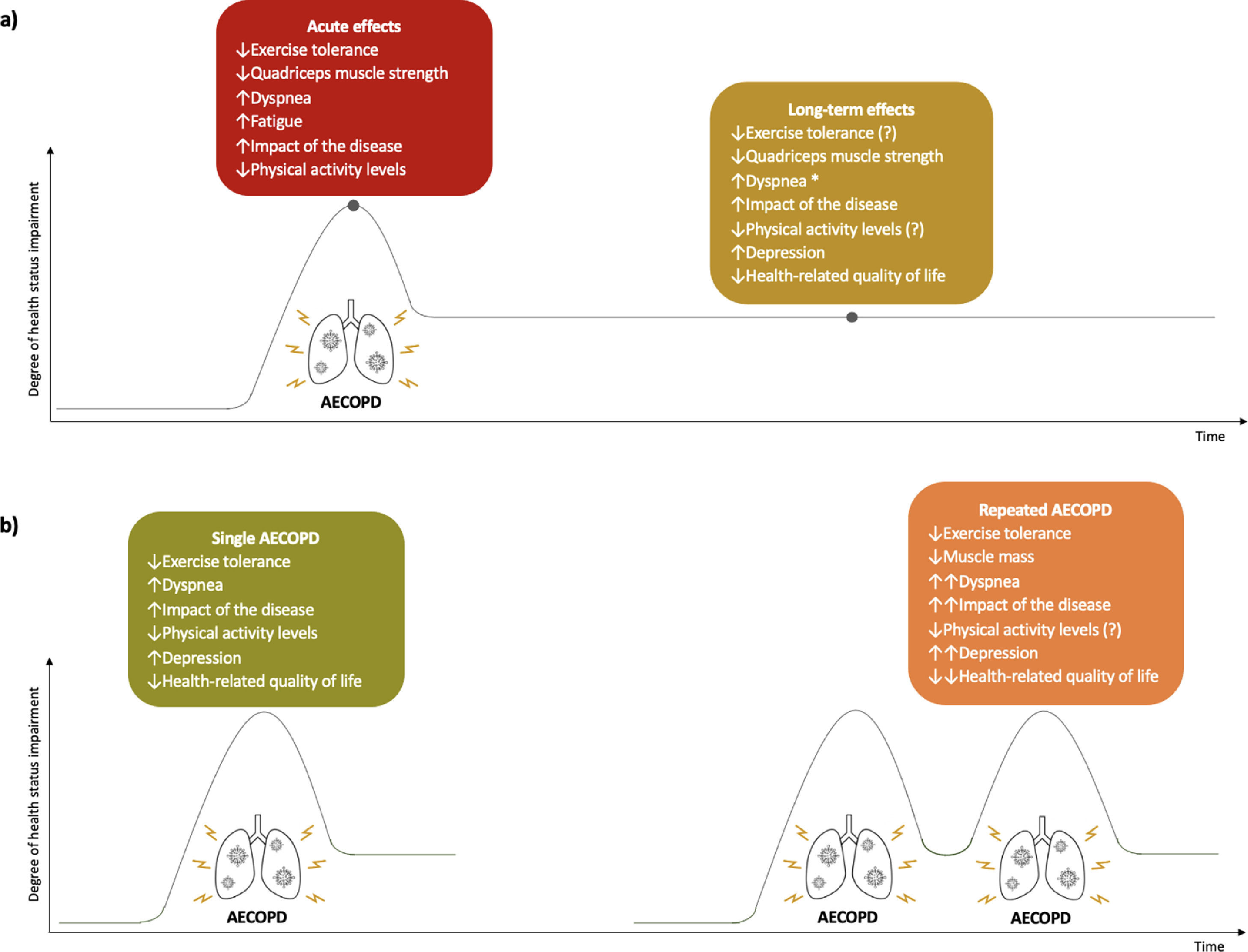
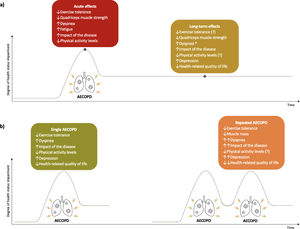
A summary of the acute and long-term effects of AECOPD and the impact of single and repeated exacerbations on health status can be found in Fig. 3.
Effects of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) on patients’ health status. (a) acute and long-term effects of AECOPD on health status. (b) effects of single and repeated AECOPD on health status. ↑, increase; ↓, decrease; ↑↑, greater increase than in single AECOPD; ↓↓, greater decrease than in single AECOPD; *, only in repeated AECOPD; (?), conflicting results found.
This scoping review summarized the acute and long-term effects of AECOPD and the impact of repeated exacerbations on the health status of patients with COPD. Acute effects included a worsening of symptoms and impact of the disease, and a reduction of exercise capacity, quadriceps muscle strength and physical activity levels. Long-term negative effects were reported on complaints, quadriceps muscle strength and quality of life. Repeated exacerbations negatively impacted FFM and resulted in further worsening of complaints (i.e., dyspnoea and impact of the disease) and QoL (i.e., health related QoL and depression). The impact of repeated exacerbations on exercise tolerance and physical activity levels is less clear.
Impact of exacerbations on complaintsAn AECOPD is defined by an acute worsening of respiratory symptoms and it is usually characterized by increased airway inflammation, mucus production and air trapping.1 A recent proposal on an updated definition of AECOPD states that these events are characterized by dyspnoea and/or cough and sputum that worsens over up to 14 days, possibly accompanied by tachypnoea and/or tachycardia, and often associated with increased local and systemic inflammation.46 Not surprisingly, an acute worsening of complaints (i.e., dyspnoea and fatigue) during exacerbations was found.24,26 During this period, the worsening of airway obstruction results in increased work of breathing, dynamic hyperinflation and hypoxaemia, leading to symptoms of dyspnoea and fatigue.47-49 These symptoms usually recover following the exacerbation,47,49 yet persistent (up to 2 years) and higher increases in dyspnoea levels were found in frequent exacerbators,26,28 emphasizing the high impact of these events on patients’ life. In fact, the impact of the disease, as assessed by the CAT, has been found higher during either single or repeated exacerbations and on the long-term,35,37-39 and associated to the worsening of lung function, systemic inflammation and functional status.50,51
Impact of exacerbations on physiological functionExacerbations result in physiological impairment. There is an immediate reduction in QMVC three and seven days after the onset of exacerbation symptoms.24 The increased systemic inflammation appears to contribute to this, as QMVC has been correlated with systemic levels of IGF-I and CXCL8 in AECOPD52; and pro-inflammatory cytokines activate pathways leading to atrophy and inflammation-induced muscle dysfunction.53,54 The reduced physical activity levels and medication used (e.g., corticosteroids) during this period seem to also play a role in QMVC reduction.53,55 Moreover, the increased cost of ventilation during exacerbations increases resting energy expenditure, with negative consequences on body weight and muscle mass,55 highlighting a potential role of nutritional support in these patients.56 Over the 1-year follow-up, one study found that frequent exacerbations were associated with FFM decline,30 possibly indicating a cumulative effect of AECOPD on muscle mass depletion – the main constituent of FFM.57 Furthermore, the same study found that the decline in FFM was also associated with the use of maintenance oral corticosteroids,30 which has been described in steroid-induced myopathy.58,59 Given the known relationship between reduced muscle mass and muscle strength impairment,58,60,61 one could expect that frequent AECOPD would also be associated with QMVC decline. Nevertheless, no association was found,30 showing that muscle mass and muscle strength are not always reduced in the same proportion.62 Further research is therefore needed to enhance our understanding on the impact of AECOPD on muscle dysfunction.
Exacerbations (single or repeated) also result in an acute decrease in functional exercise tolerance, which was expected since the breathing load is acutely increased and patients experience breathlessness even when performing low-intensity activities.47,63 Moreover, fatigue and quadriceps muscle weakness also play a role as limiting factors of exercise performance.64 Surprisingly, although it is often assumed that AECOPD leads to a permanent impairment on exercise performance, it is still unclear if the decrease in exercise tolerance recovers after a few days,24 alongside with symptomatic recovery,6,47,65 or whether it is sustained on a long-term, with studies26,28 even suggesting a sustained impairment 2 years after the exacerbation. It is known that (i) different types of AECOPD result in distinct clinical findings, prognosis and responses to treatment66,67; (ii) hospitalized patients with AECOPD are the ones presenting worse prognosis68; and (iii) the use of antibiotics and corticosteroids presents inconsistent benefits depending on the clinical setting and severity of AECOPD.68 Since in one study24 only community managed AECOPD were included and patients were mostly treated with a combination of antibiotics and oral corticosteroids, while the other two studies26,28 involved a percentage of AECOPD that resulted in hospital admission and did not report the exact number of patients treated with each medications, it is likely that these factors may have contributed to the disparity of the findings. Moreover, the six-minute walk test presents a significant learning effect and evidence shows the necessity of conducting the test twice in AECOPD.69,70 Therefore, differences in the methodology regarding the frequency and timing of assessments and the number of tests performed, might have also contributed to the inconsistency in the results. Future studies with robust methodologies are needed to clarify the long-term effects of AECOPD on exercise tolerance.
Impact of exacerbations on functional impairmentExacerbations lead to functional impairment observed by reduced physical activity levels,24,25,27,42 which seems to be more accentuated in repeated AECOPD. An acute decrease in physical activity levels is associated with the severe inactivity and low amount of time spent in weight-bearing activities during hospitalization for AECOPD, general immobility and tendency to become housebound.16,71,72 The worsening of symptoms - particularly dyspnoea at rest - hypoxaemia, muscle weakness and loss of exercise capacity might also reduce physical activity levels.71,73,74 In turn, reduced physical activity levels lead to further skeletal muscle deconditioning and reduction of exercise capacity, bringing patients into a vicious cycle of symptoms and inactivity.74-77 It has been hypothesized that this vicious cycle could explain the long-term effect of AECOPD on physical activity levels.74 Nevertheless, conflicting evidence was found,74,78 with studies showing that physical activity levels can either recover in a few days/weeks or may not return to pre-exacerbation levels, especially in the case of frequent exacerbators.25,27,40,42,79 The differences in the timepoints of assessment and outcome measures used (e.g., objective vs. subjective measures) might have contributed to the heterogeneity in the results found.80 Further studies, following the international recommendations on how to measure physical activity in patients with COPD,81 are needed to better understand the impact of AECOPD on physical activity over time.
Impact of exacerbations on quality of lifeThe effects of exacerbations on QoL were the most studied. A long-term decline (up to 8 years) in QoL due to AECOPD was found,27,29,33,34,43,45 which was more pronounced in frequent exacerbators,28,29,31,33-35,43-45 suggesting a cumulative effect of repeated exacerbations in this domain. It is known that AECOPD have a huge impact on patients' everyday activities (e.g., walking, sleeping, work) and, consequently, they feel unable to maintain their lifestyle and make plans.82-84 Given these reasons and all the consequences of AECOPD mentioned above on complaints, physiological functioning and functional impairment, the decline on QoL was expected.
Implications of the findings for researchIn sum, heterogeneity amongst the presentation and trajectory of recovery of exacerbations was found, and there is at least a subset of patients presenting a sustained worsening of health status after (repeated) exacerbations. This heterogeneity might have been influenced by the variety of AECOPD definitions found, as it is known that even small changes in the definition used affect the incidence rate, type and classification of exacerbations, with event-based AECOPD being usually considerably less identified and in specific groups of patients.23,85,86 The underlying cause of the AECOPD (e.g., viral exacerbations are known for being more severe and taking a longer recovery time),87 its severity, treatment setting (e.g., hospital vs. home), the standard of care provided (i.e., pharmacological treatment), presence of comorbidities, socioeconomic status and/or knowledge about the disease, might have also contributed to the heterogeneity found.67,87-93 A more accurate definition of AECOPD and understanding of its aetiology and diagnosis is, therefore, critical to better recognise exacerbations’ clinical impacts and improve treatment strategies. Despite this heterogeneity, AECOPD are usually treated uniformly (i.e., bronchodilators, systemic corticosteroids and/or antibiotics) without considering the different underlying outcomes and treatment needs.94 Since one size does not fit all, comprehensive health status assessments that allow the identification of distinct treatable traits amongst individuals are crucial to personalize treatments, contributing to improved AECOPD recovery and prevention.95 In this review, we have found that no study assessed all the domains that compose health status, thus future studies should explore the effects of AECOPD on patients’ health status using comprehensive measures. Moreover, early pulmonary rehabilitation is a safe intervention for the management of patients with AECOPD that has been shown to improve QoL, and reduce the length of hospitalization, hospital readmissions and mortality in these patients, while targeting several treatable traits (e.g., physical activity, exercise capacity, muscle weakness, dyspnoea) that are associated with exacerbation recurrence.4,96-98 Evidence suggests that pulmonary rehabilitation may be offered to patients with AECOPD to recover their pre-exacerbation health status.99 Pulmonary rehabilitation seems particularly important to those presenting a late recovery or who never recover to pre-exacerbation levels.90 Future studies should focus on personalizing pulmonary rehabilitation programs to target the different identified treatable traits during AECOPD.94,95
Methodological considerationsThis scoping review has some strengths and limitations that need to be acknowledged. To our knowledge, this is the first review of the impact of AECOPD in the different sub-domains of health status in patients with COPD. A thorough search and screening were performed, and rigorous methodological and reporting frameworks (JBI and PRISMA-ScR) were followed. Nevertheless, we did not publish a protocol of the study before conducting this scoping review, thus the methods were not peer-reviewed prior to our search. Several concepts have been used to define health status and numerous tools were designed to assess the different aspects of this comprehensive measure. To ensure clarity, this work followed a previously published assessment framework for health status in patients with COPD.18 Additionally, the summary of the impacts of AECOPD on the different domains of health status was challenging due to the diversity in exacerbation definition and diagnosis, timepoints of assessment, outcome measures used, and lack of clarity of some results found. Most studies included a population combining different AECOPD severities, treatments and/or treatment settings, which prevented the presentation of these results separately. All these aspects have hampered results synthesis. Lastly, since most of the studies included were focused on moderate to severe AECOPD, the findings of this review cannot be translated to mild AECOPD.
ConclusionExacerbations of COPD result in both acute and long-term impairments in all health status domains. Acutely, there is a worsening of symptoms and impact of the disease, and a reduction in exercise capacity, quadriceps muscle strength and physical activity levels. Long-term negative effects are noticed on complaints, quadriceps muscle strength and quality of life. Repeated exacerbations result in a reduction of FFM and further worsening of complaints and QoL. However, the impact of repeated exacerbations on exercise tolerance and physical activity levels, and the trajectory of patients' recovery, is less clear due to the lack of studies and conflicting evidence found. Future research focused on these aspects is therefore warranted.
FundingThis work was funded by Programa Operacional de Competitividade e Internacionalização – POCI, through Fundo Europeu de Desenvolvimento Regional - FEDER (POCI-01–0145-FEDER-007,628), Fundação para a Ciência e Tecnologia (SFRH/BD/147,200/2019) and under the project UIDB/04,501/2020. In addition, Ana Machado's work is supported by Bijzonder Onderzoeksfonds – Bilaterale Samenwerking (BOF BILA) from Hasselt University (BOF BILA reference: DOC/SCHL-BOSE/190/522). Jana De Brandt is funded by the Flemish government through the Research Foundation – Flanders (FWO) grant #11B4718N. Sarah Haesevoets is supported by Bijzonder Onderzoeksfonds (BOF) from Hasselt University (BOF reference: BOF21KP15).