Lung transplantation is an established treatment for end-stage lung diseases such as fibrotic interstitial lung diseases (ILD) and chronic obstructive pulmonary disease which are both the most common indications for lung transplant.1,2
Despite extensive pre-transplant evaluation, neoplasm can be found in explanted lungs with an incidence ranging between 0.8% and 2.2%,3 for several reasons. The differential diagnosis between imaging features associated with pulmonary fibrosis such as fibrotic nodules, ground-glass opacities or consolidations may be difficult to distinguish from neoplastic nodules and invasive diagnostic approaches may not be recommended due to associated risks such as pneumothorax and worsening respiratory failure.1,3 The incidental finding of neoplastic diseases raises difficult issues in management due to the need for immunosuppressive therapy with unpredictable effects on disease progression, interactions with chemotherapy and other oncology treatments and with increased risk of infection and other complications.
We describe a clinical case in which the unexpected finding of adenocarcinoma in the explanted lungs poses delicate management problems.
Clinical caseWe present a case of a 44-year-old woman, former smoker for 10 years (21 pack-years) with previous contact with birds during six years. The patient was referred to a Diffuse Lung Diseases clinic due to progressive fatigue, worsening in the previous two years, with exertional dyspnoea (modified Medical Research Council [mMRC] — 2); no other respiratory symptoms were noticed. A thoracic computed tomography (CT) showed a diffuse pulmonary cystic disease suggesting advanced Langerhans cell histiocytosis (Fig. 1). Blood tests were unremarkable. Pulmonary functional tests revealed a mild pulmonary restriction (FEV1 68% of predicted, FVC 68% of predicted, FEV1/FVC 86%, TLC 64% of predicted) and a marked decrease in DLCO (16% of predicted, increasing to 20% when corrected to the alveolar volume). The 6-minute walk test showed desaturation to 62% and a walking distance of 260 meters. The histology from surgical lung biopsy confirmed hypersensitivity pneumonitis (HP) and pulmonary Langerhans cell histiocytosis (PLCH).
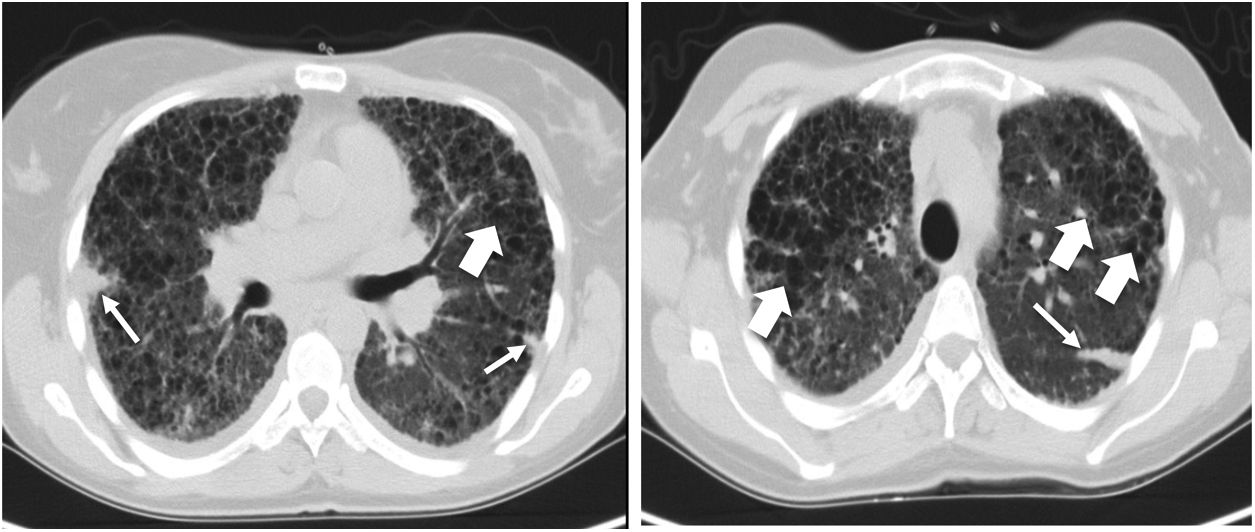
Pre-transplant computed tomography of the chest in lung window showing a diffuse change in the pulmonary architecture with multiples cists, some of them confluent with thin walls (large arrows) in relation with Langerhans cell histiocytosis. It is also described a septal thickening due to confluence of fibrotic areas with formation of some nodules (thin arrow).
In spite of immunosuppressive treatment with prednisolone (10mg/day) and mycophenolate mofetil (MMF), she presented clinical, functional and radiology worsening and was referred for lung transplant two years after the initial diagnosis.
A year and a half later, she underwent a bilateral lung transplant under extracorporeal membrane oxygenation (ECMO). Histopathology analysis of explanted lungs identified advanced pulmonary fibrosis and multi-focal and bilateral nodules of adenocarcinoma with solid and acinar pattern, positive for TTF-1 and CK7 with focal invasion of the visceral pleural. Post-transplant staging through 18-flour-deoxyglucose positron emission tomography (18-FDG-PET) did not suggest evidence of distant metastasis. Two-months later, due to severe back pain, a hip-CT showed a lytic lesion in the sacrum with joint bone destruction and cortical disruption. The 18-FDG-PET revealed a hyper-metabolic focus (maximum SUV 11.6) in the same location. The tumor was then classified according the American Joint Committee on Cancer (AJCC) 8th edition as pT4N0M1b (stage IV). Molecular characterization showed 10% of tumoral cells expressing the PD-L1 receptor and only the not targetable mutation [c.34G>T (p.Gly12Cys)] in the KRAS gene (exon 2) was detected by Next-Generation-Sequencing (NGS).
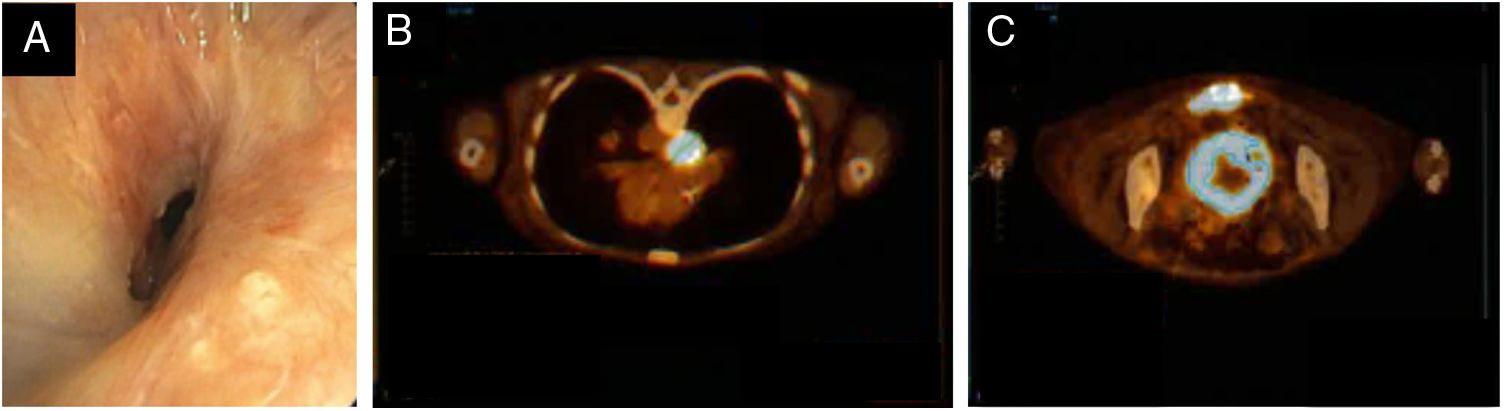
The patient never met clinical conditions for systemic neoplastic treatment due to recurrent respiratory infections, which were further complicated by progressive stenosis of the right bronchial anastomosis despite endoscopic dilation attempts (Fig. 2A). She underwent stereotactic radiotherapy on the bone lesion for palliative pain control and was referred to the palliative care unit. Despite all efforts, she had an unfavorable course with documented progression of the oncological disease with progressive enlargement and metabolic increase of bone involvement (SUV 21.9) and with emergence of voluminous infra-carinal adenopathy of high metabolism (SUV 15.7) (Fig. 2B,C).
She died 12 months after lung transplant due to septic shock and respiratory failure.
DiscussionThe current case shows that distinguishing fibrotic interstitial lung diseases (ILD) from lung cancer can be a diagnostic challenge. The prevalence of lung cancer in interstitial pulmonary fibrosis (IPF), the most common ILD, ranges from 2.7% to 48%,4 significantly higher than in general population. It is recognized that both entities share multiple common genetic, molecular and cellular processes. Some studies also describe a preferential distribution of tumors in lower lobes suggesting a relationship between fibrotic areas and cancer development, a phenomenon called as “scarcinoma”, although it is premature to prove a direct association with this finding.5
PLCH is known as an uncommon interstitial lung disease in young adults with an unpredictable course that may be associated with an increased susceptibility to the development of malignant neoplasms.6 Lung cancer is the most common solid organ malignancy occurring in approximately 5% of PLCH patients.7 An interesting feature related to the pathogenesis of PLCH is the presence of a recurrent BRAFV600E mutation present in almost 50% of LCH lesions.8 This mutation is also found in other different tumors9 supporting the association between PLCH and neoplastic diseases. Smoking habits are another recognized potential risk factor for both PLCH and lung cancer. It is estimated that more than a half of PLCH patients have previous or current smoking habits.9
Since an active neoplasm is an absolute contra-indication for lung transplant, its diagnosis during pre-transplant evaluation is crucial.10 Patients with fibrotic ILD and solid pulmonary nodules are challenging to approach. There are no protocols to guide the surveillance of such nodules: CT screening is recommended when the nodules are inferior to 8mm, and PET-CT should be performed when the nodules are over 8mm with a low or moderate pretest probability of malignancy.5 Endobronchial ultrasonography with transbronchial needle aspiration is one of the diagnostic techniques to be considered for histological sampling in patients with high pretest probability of malignancy.2 In our particular case, during the time on waiting list for transplant, annual thoracic CT showed progression of disease to pulmonary fibrosis with pulmonary nodules that remained stable over time. Evaluating retrospectively, some of those nodules could have been of neoplastic origin, however the invasive diagnostic approaches available at that time were extremely risky for the patient due to the possible fatal complications, namely respiratory failure.
An interesting aspect of this case is the disagreement between the initial and post-transplant histological diagnosis. As far as we know, there are no published papers analyzing the histological transformation that can occur after lung transplant, despite descriptions of some ILDs evolving to pulmonary fibrosis over time. An unpublished retrospective study demonstrated that in the majority (80.5%) of patients submitted to lung transplant due to ILD their explanted lungs showed a histologic pattern of usual interstitial pneumonia (UIP), although only one third of them had previous diagnosis of IPF.11 This finding suggests a fibrotic evolution between initial diagnosis and lung transplant in non-IPF patients, which is consistent with the finding of pulmonary fibrosis on the resected lungs from our patient.
Another increased risk factor for neoplastic disease is the immunossupression that was started some years before the lung transplant. MMF is inclusively described as being related to tumor progression, possibly due to various mechanisms, including disruption of apoptosis and DNA repair.3 After lung transplant, managing immunosuppressive therapy was the most challenging approach. It was decided to discontinue MMF and remain with tacrolimus and prednisone at the lowest limit of the desired range.
This case offers some noteworthy learning points. The increased risk of primary lung cancer in lung transplant candidates must be acknowledged. Certain imaging features, including pulmonary nodules or masses, should be followed or biopsied, depending on how advanced the ILD is and the urgency for transplant. Patients with ILD should undergo bronchoscopy lavage for cytological samples. It is important to have a high level of suspicion for neoplastic disease since the clinical scenario does not always point to this differential diagnosis. This is reflected in the present case, as the patient had only a remote smoking history and had no on-going weight loss or other signs that would suggest a concurrent malignant disease. This case also reinforces the need for research for noninvasive blood tests that could identify neoplasms. These tests would be extremely useful in such challenging clinical situations, once the invasive alternative diagnostic approaches they have are highly risky.
Conflicts of interestThe authors have no conflicts of interest to declare.