Severe eosinophilic asthma is a clinical phenotype of asthma; the underlying mechanism is an eosinophilic inflammatory pattern in the airway, characterized by recurrent exacerbations and poor disease control. Both in atopic and non-atopic subphenotypes, IL-5 plays a major role across the pathway of eosinophilic inflammation.1
Pharmacological agents targeting IL-5 have shown to significantly reduce severe exacerbations and oral corticosteroids (OCS) use in severe eosinophilic asthma patients, particularly in those with higher eosinophilic count and a history of frequent exacerbations.2,3 Mepolizumab is an anti-IL5 monoclonal antibody, approved as an add-on therapy in both allergic and non-allergic patients with severe eosinophilic asthma, and recommended on step 5 of the 2022 GINA guidelines.4
Despite the continuous decrease in asthma mortality, severe asthma exacerbations still represent the highest contribution for all-cause mortality during the first month following the event.5
Therefore, this report addresses a potential additional role of anti-IL5 in treating acute refractory severe asthma exacerbations and reduce its mortality. We report an off-label use of mepolizumab in a patient without previous maintenance treatment, during a life-threatening asthma attack.
A 25-year-old female presented to the emergency department complaining of severe dyspnea, wheezing and cough, with progressive worsening in the previous 4 weeks. The patient had history of allergic asthma without any maintenance therapy, having abandoned anti-asthmatic treatment during childhood. The patient did not mention any comorbidities, medication, or smoking habits, nor any asthma related exacerbations.
On admission the patient was restless, showing signs of respiratory distress, and decreased pulmonary sounds. Chest x-ray showed parenchymal hyperinflation with no signs of infiltrates. Blood gas assessment showed severe hypoxemia, normocapnia and no other alterations. Given the persistent signs of tachypnea, paradoxical breathing, and high oxygen requirements, the patient was promptly admitted to a respiratory ICU, and initiated on invasive mechanical ventilation. In the following hours the patient evolved with refractory bronchospasm and severe blood gas deterioration with pH 6.85 and carbon dioxide partial pressure (PaCO2) 145 mmHg, under pressure-regulated volume control-mode (settings: tidal volume 380 mL; respiratory frequency 22 cycles per minute; positive end-expiratory pressure, PEEP 0 mmHg, inspiratory to expiratory ratio 1:4). Blood tests at admission and prior to corticosteroid administration revealed blood eosinophils of 11% (2680/µL) and no other alterations. In the subsequent days, and despite adjusted ventilatory settings, deep sedation, analgesia, muscle paralysis and anti-asthmatic and bronchodilator therapy [inhaled salbutamol (200 mcg q4h), ipratropium (80 mcg q4h) and beclomethasone (500 mcg q8h), IV methylprednisolone (125 mg q6h – withdrawal after 6 days), IV aminophylline (240 mg q12h), IV magnesium sulphate (2 g q12h), IV salbutamol (5–10 mcg/min intermittently during 3 days), IV ketamine (0.5–1.25 mg/kg/h intermittently during 5 days)], mechanical ventilation remained a challenge, considering dynamic hyperinflation, high auto-PEEP values (maximum 12 cmH2O), and high peak inspiratory pressures. No CT scan or bronchoscopy was performed in the acute phase due to the hemodynamic instability and the absence of infiltrates in the chest x-ray. Eosinophilic granulomatosis with polyangiitis (EGPA) was not fully ruled out; however, no symptoms of ear, nose, and throat involvement nor systemic vasculitis manifestations were present, and serum cANCA were negative.
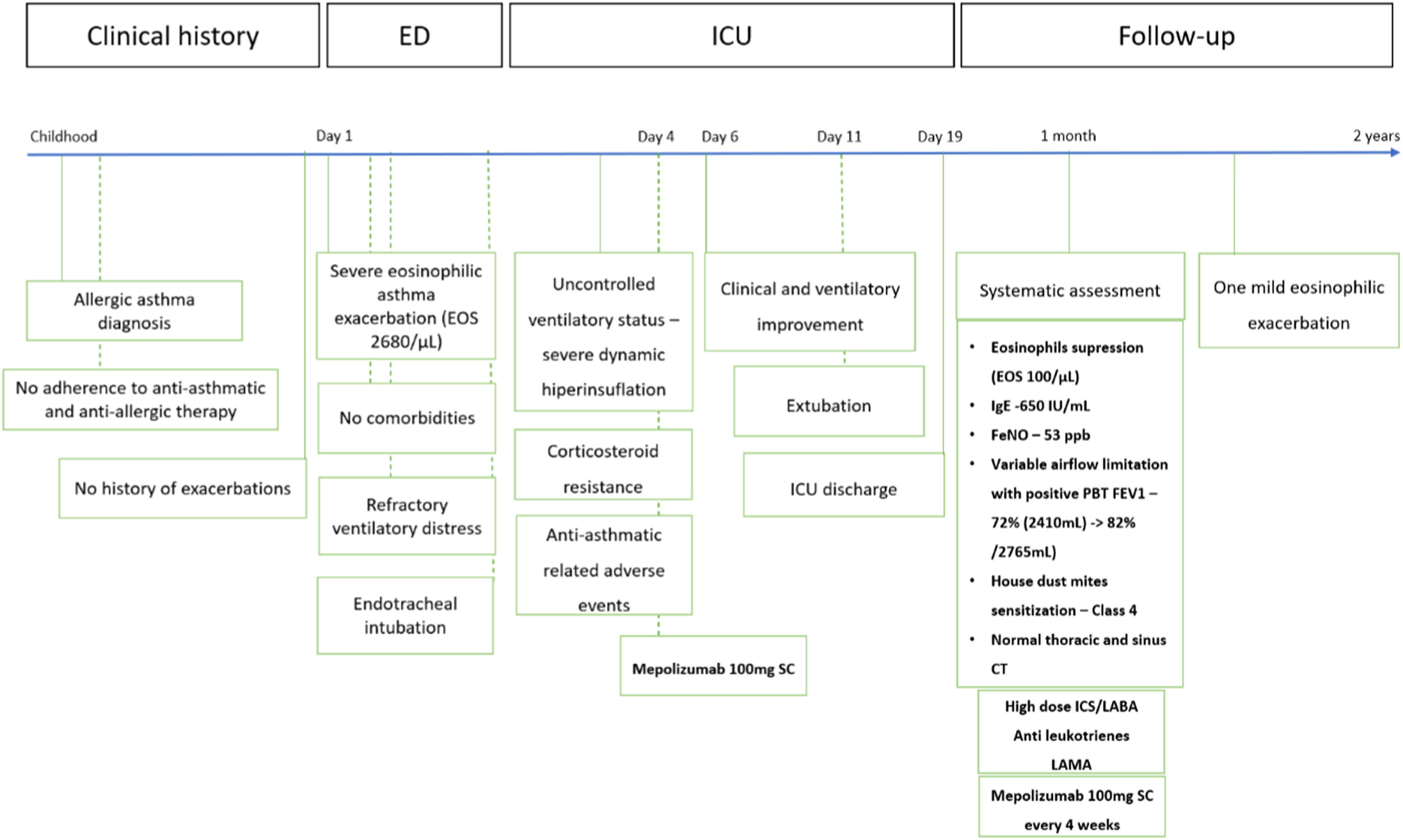
On the 4th day of ventilatory support,100 mg mepolizumab was administered subcutaneously, as an off-label last-resort attempt to recover from the critical ventilatory state. After 48 h of mepolizumab injection, we observed a clinical improvement, normalization of PaCO2, peak pressures, and residual auto-PEEP. Then, the patient started IV corticosteroids tapering, and was weaned from mechanical ventilation and extubated on the 11th day of ventilation. No minor or serious adverse effects were registered. The timeline of the patient's clinical evolution is shown in Fig. 1.
Case report timeline following the CARE guidelines.
Abbreviations: EOS – eosinophils; CT – computerized tomography; ED – emergency department; ICU – intensive care unit; FeNO – fraction of exhaled nitric oxide; FEV1 – forced expiratory volume in first second; PBT – post bronchodilation test; SC – subcutaneous; ICS – inhaled corticosteroid; LABA – long-acting beta-agonist; LAMA – long-acting muscharinic antagonist.
The patient was discharged from the ICU after 19 days. At 1-month follow-up, systematic assessment revealed variable airflow limitation with a FEV1 of 72% (2410 mL) and FVC of 89% (3420 mL), a positive post-bronchodilation test, with FEV1 reaching 82% (2760 mL), high serum total IgE levels 650 IU/mL, high fraction of exhaled nitric oxide (FeNO) 56 ppb, and sensitization to house dust mites. Eosinophilic count was depleted (1% – 100/µL), and thoracic and sinus CT were normal. Clinical improvement was documented through validated quality-of-life questionnaires related to rhinitis – Self Assessment of Allergic Rhinitis and Asthma (SACRA) and asthma – Asthma Quality of Life Questionnaire (AQLQ). The Asthma Control Test at 1-month follow-up documented symptomatic improvement (ACT-19). The patient maintained follow-up at the severe asthma outpatient clinic, and has been under inhaled treatment with high dose ICS/LABA, LAMA, anti-leukotrienes and mepolizumab for two years, with present adequate asthma control (ACT-22) and only one documented mild eosinophilic exacerbation.
Although its outcomes have been studied for medium/long term, pharmacokinetic studies with mepolizumab identified pronounced and maximal reductions in eosinophil count 3–4 days after infusion and estimated at ∼85% relative to baseline, with a single administration.6,7 This short-term benefit, together with a vast local and multicenter experience regarding its safety and effectiveness as a corticosteroid-sparing agent, guided its choice over other anti-IL5 agents.3
Other studies have previously reported the use of mepolizumab, reslizumab and omalizumab in severe asthma exacerbations, also with similar degrees of success.8–10 Benralizumab, a monoclonal antibody against IL5‐Rα, has also proven to induce a rapid response to treatment during exacerbations of severe asthma, with peak flow improvements after four days of administration,11 eosinophil count suppression, and 19% increase in FEV1 after 48 h.12 We posit that, in this case, the observed objective improvement in the ventilatory mechanic pressures 48 h after mepolizumab can be interpreted as an equivalent outcome.
Starting mepolizumab during an exacerbation and continuing it after follow-up assessment represented a unique approach, with short and long-term benefits. The early response to mepolizumab proved to be crucial in limiting the eosinophilic inflammatory pathway, allowing for a faster corticosteroid withdrawal, and being of paramount importance in reversing a critical ventilatory state, refractory to high dose corticosteroids and bronchodilators. Its off-label use has also revealed a good safety profile.
The main limitation of our case is the deficient phenotype assessment of the previous asthma diagnosis. We also could not fully rule out other diagnoses of eosinophilic also disorders such as EGPA; however, mepolizumab 100 mg every 4 weeks has also been shown to be effective in controlling respiratory manifestation of EGPA.13
This case supports the hypothesis of a novel role for mepolizumab in acute settings in refractory severe exacerbations of eosinophilic asthma.
Ethical considerationsWritten permission for off-label use was obtained from the legal representative of the patient.
This study was retrospectively approved by the Institutional Ethic Committee.
Informed consentAppropriate written informed consent was obtained from the patient for the publication of this case report.