A1Antitrypsin (AAT), the major protease inhibitor in serum and severe AAT deficiency (AATD) worldwide, relates mainly to the homozygous state of the PI*Z variant.1 However, the genetic repertoire of severe AATD is constantly expanding far beyond the homozygous PI*Z variant to a multitude of rare alleles decoding for deficient, dysfunctional or non (null-alleles) producing AAT.2 In recent years a geographical trend towards South Europe also began to appear regarding severe AATD related to rare variants.3 Recently, we described that by genotyping AATD in Greece, a multiplicity of rare and ultra-rare variants and a diversity of rare combinations were observed in two-thirds of patients, confirming an established North-South European geographical trend in rare variants.4 The Greek rare variants embraced the null PI*Q0Bellingham, PI*Q0Amersfoort, PI*Q0Granite Falls, PI*Q0Saint-Etienne, PI*Q0Mattawa; and the deficient variants PI*MHeerlen, PI*MProcida, PI*MMalton, PI*MWürzburg, and PI*NHardfordcity; PI*OFeyzin and PI*PLowell.(p.Asp280Val).4 The epitome of rarities in AATD in Greece was the discovery of a novel variant named as Q0Attikon(c.1A>G; p.Met1?), herein described in detail.
A 56 year-old non-smoker male, ΒΜΙ=27.1 kg/m2, with no family history of lung disease or toxic environmental and occupational exposures was referred to our center for repetitive exacerbations upon overlapping early-age-emphysema, bronchiectasis and eosinophilic asthma. He had dyspnea on exertion which had progressively deteriorated in the last 3 years (MRC=3) and cough. The patient had severe panlobular emphysema with bronchiectasis (Fig. 1a,b,c), de-oxygenated on exertion from SpO2=94% to 80% and presented values of forced expiratory volume at 1 sec (FEV1)% predicted, FEV1/ Forced vital capacity (FVC) %, diffusion capacity of the lung for carbon monoxide (DLCO)% predicted and transfer coefficient for the diffusion of carbon monoxide (CO) [DLCO/alveolar volume (VA)] % predicted of 37, 34.8, 53 and 72 respectively. More precisely in the last 2 years, the FEV1 value deteriorated from 1520 ml to 1300 ml, corresponding to an accelerated decline of 110 ml per year. AAT serum levels by nephelometry were 0.14 g/L (0.9–2.0 g/L) with normal values of CRP at 2.7 mg/L(0–5 mg/L).
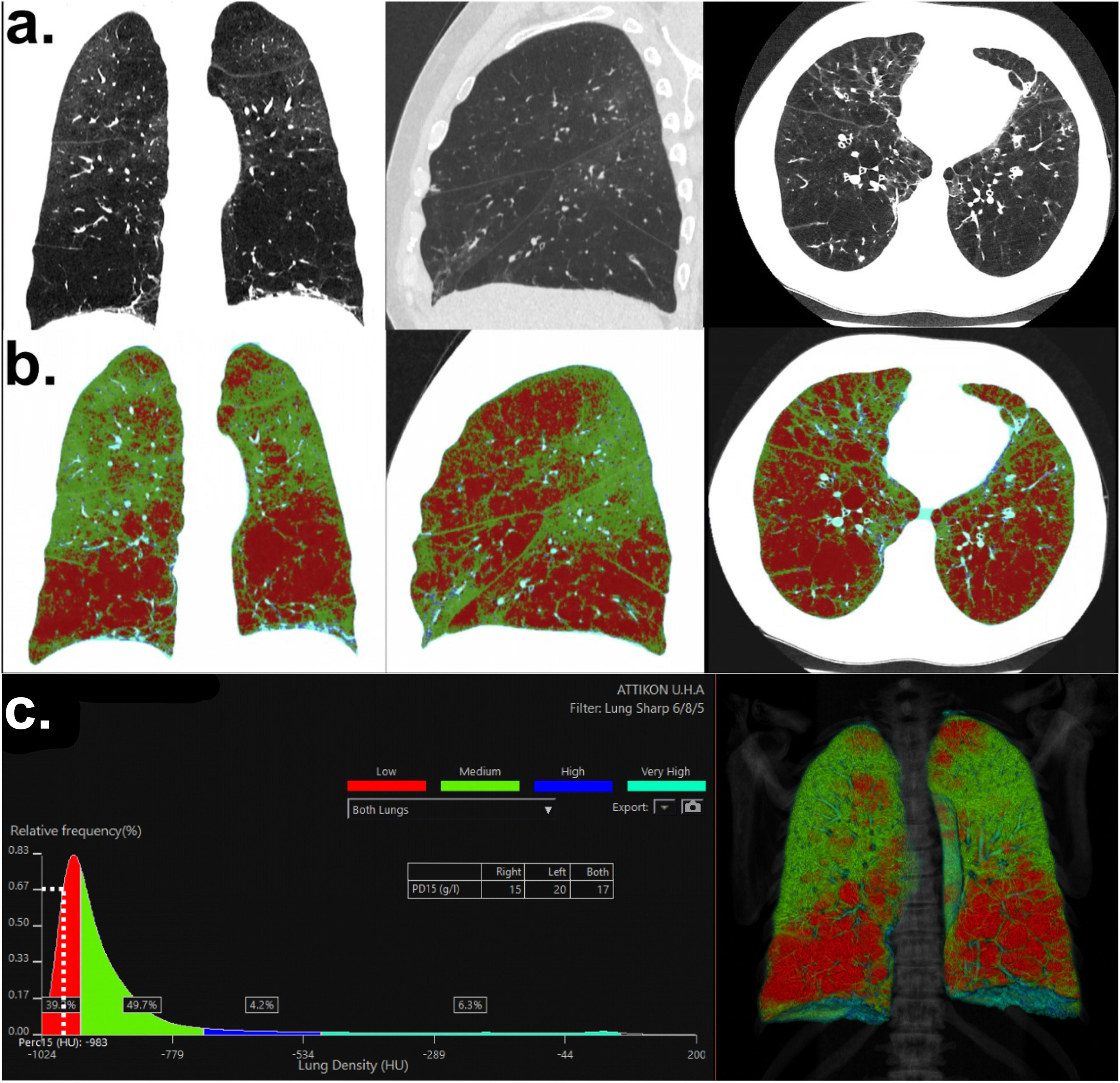
a. High-resolution chest computed tomography of the proband. Normal lung parenchyma distortion due to emphysema. Frontal, sagittal and axial views (from left to right), b: Color-coded visual map on lung density analysis to assess lung emphysema. Red-colored pixels represent attenuation lower than <-950HU, c: Lung density distribution graph. PD15 = -983HU shows an estimated pulmonary density of 17 g/L (for both lungs). The percentage of emphysema (areas of low-attenuation) was estimated ∼ 44,2% for both lungs.
Genetic analysis was performed at the German AAT Laboratory at the University of Marburg. The laboratory methods are described in detail elsewhere.4 After informed consent, the patient's dried blood spot samples were tested using the Progenika AAT genotyping kit (Progenika Biopharma, S,A, Derio, Spain) on the Luminex 200 (Luminex, Austin, TX, USA). This multiplex PCR (polymerase chain reaction) and hybridization test allows the simultaneous identification of 14 alleles. The test confirmed the presence of Z allele in heterozygosity. Phenotyping was perfοrmed by isoelectric focusing (IEF). IEF is used for samples that indicate other mutations may be present. On IEF only the Z-protein was identified, suggesting an additional null mutation given the discrepancy between the very low levels of AAT and the genotype- phenotype findings so far. Whole SERPINA1 gene sequencing in a reference laboratory in Spain followed identifying a (c.1A>G) heterozygous mutation. This new variant, identified by Next Generation Sequencing (NGS) and confirmed by Sanger sequencing (Fig. 2), affected the translation initiation codon (Met1) completely inhibiting AAT production. The case was discussed in a web-based multidisciplinary meeting dedicated to AATD (www.respifil.fr) confirming the above findings. Family members were investigated (Fig. 2) and augmentation therapy was recommended. The patient presented no findings compatible with liver disease both in liver biology blood examination and in hepatic ultrasound and shear wave elastography (S0 steatosis and F0 fibrosis staging) and had no history of alcohol abuse.
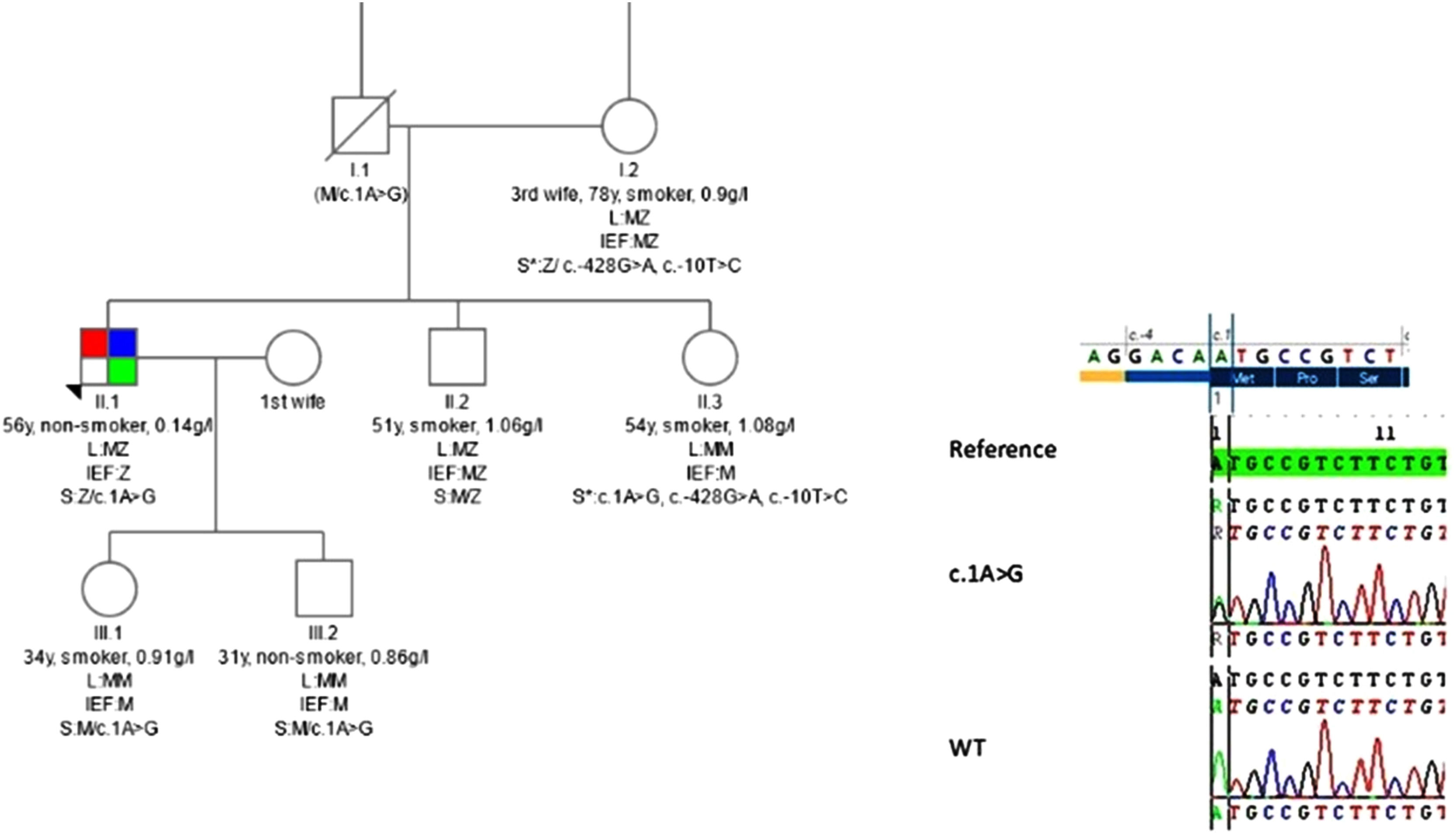
Three-generation pedigree of the proband. Numbers below individual symbols are age at inclusion in the study. Smoking status (pack/years) and levels of alpha-1 antitrypsin in serum (g/l) are also depicted. In details shown for each individual [parents I1, I.2, the proband, sister and brother II.1, II.2, II.3 from the third marriage of the father of the proband and children III.1, III.2 first marriage of the proband], the results of the analysis including LUMINEX (L), Isoelectric focusing (IEF) and gene sequencing (S). *The variant c.-428G>A; c.-10T>C found in individuals I.2 and II.3 seems to be like a M variant in the IEF. c.1A>G corresponding to Q0Attikonnewly described mutation is depicted at the proband II.1 in compound heterozygous state with PI*Z as well as at his sister II.3 and his children III.1 and III.2 at heterozygous state. (M/c.1A>G) for individual I.1 indicates an obligate carrier status. All members of the bloodline are reportedly asymptomatic except the proband which presents emphysema (red), bronchiectasis (blue) and asthma (green). In the insert theQ0Attikon mutation confirmed by Sanger sequencing: sequencing results of proband compared with wild type and reference sequence. Translation initiation codon (Met1) is affected by this mutation (top of the insert figure).
The new variant was named by the University-Clinic and Hospital of discovery Pi*Q0Attikon. In this case the new variant proved clearly pathogenic and seriously deleterious. In the absence of functional studies, this was demonstrated mostly from a high REVEL score of 0.759 (range 0–1)5 in association with the clinical presentation of the patient, a never smoker. We have shown that in Greece the great majority of patients with severe AATD relates to rare variants instead of the Pi*ZZ phenotype that prevails worldwide.4 Therefore, the discovery of a novel, never reported and clearly pathogenic variant constitutes the epitome of rarities in severe AATD in Greece.
Rare variants are increasingly reported in recent years, by genotyping, mainly in south Europe and surprisingly as reported by us in Greece, the epicenter of rarities in severe AATD.2,4,6 A significant proportion were homozygous Q0 variants in a multiplicity of different genotype combinations with very low AAT levels, almost imperceptible in fact. Another significant proportion of variants were in compound heterozygous state with the Z variant as in the case described herein and another significant proportion were Mdeficient variants in different combinations with null (Q0) or Z variants not always identifiable without genotyping.4 From the above observation concern may arise regarding the dose-effectiveness of current dose recommendation of augmentation treatment in zero or almost zero carrying AAT levels, since previous international protocols included exclusively ZZ homozygous phenotypes. Furthermore, additional investigation is necessary regarding the clinical history and fate of these patients as well as the clinical phenotype expressed from carriers of rare variants; a project that fulfills the European Alpha-1 Research Collaboration (EARCO) consortium.7
In conclusion, the characterization of a new null variant of SERPINA1 named by the University-Clinic and Hospital of discovery Pi*Q0Attikon associated with a Pi*Z variant, leading to severe AATD is described. This rare mutation c.1A>G has never been identified before. Gene sequencing was necessary for genetic diagnosis. In the future the detection of rare genotypes by widening AATD spectrum and geographic distribution of variants may add to understanding of the anthropologic evolution of its mutations and probably help to personalize preventive and therapeutic measures.
Author's contributionsSAP made a major contribution to the concept and design of the study, to the acquisition, analysis and interpretation of data, and wrote the final version of the manuscript with EDM; MV performed the genetic analysis of the patient, made a major contribution to the interpretation of data regarding the new variant and wrote part of the manuscript; AL had major role in the clinical management of the patient, the acquisition and interpretation of data for all family members and critically revised this work for important intellectual content; MB, CL, MD, MFO made major contributions to the interpretation of data for the new variant and revised this work critically for very important intellectual content; EE, LO performed the NGS for the identification of the new variant, provided the figure for Sanger analysis and revised this work critically for important intellectual content; MK, VA had a major role in the management of the patient upon hospitalization and revised this work critically for important intellectual content; SP, CK prepared the radiology figures and figure legends of the manuscript and revised this work critically for important intellectual content; LK, IF, JFM had major contribution in the interpretation of data for the new variant and revised this work critically for very important intellectual content; CVF and TG had major contribution in the genetic analysis of the patient in their expert laboratory, played a major role in the interpretation of all data and revised this work critically for important intellectual content; EDM made a major contribution to the concept and design of the study, to the acquisition, analysis and interpretation of data, had access to all data, supervised the accuracy and integrity of all parts of the work and wrote the final version of the manuscript with SAP. All authors read and approved the final version of the submitted publication.



![Three-generation pedigree of the proband. Numbers below individual symbols are age at inclusion in the study. Smoking status (pack/years) and levels of alpha-1 antitrypsin in serum (g/l) are also depicted. In details shown for each individual [parents I1, I.2, the proband, sister and brother II.1, II.2, II.3 from the third marriage of the father of the proband and children III.1, III.2 first marriage of the proband], the results of the analysis including LUMINEX (L), Isoelectric focusing (IEF) and gene sequencing (S). *The variant c.-428G>A; c.-10T>C found in individuals I.2 and II.3 seems to be like a M variant in the IEF. c.1A>G corresponding to Q0Attikonnewly described mutation is depicted at the proband II.1 in compound heterozygous state with PI*Z as well as at his sister II.3 and his children III.1 and III.2 at heterozygous state. (M/c.1A>G) for individual I.1 indicates an obligate carrier status. All members of the bloodline are reportedly asymptomatic except the proband which presents emphysema (red), bronchiectasis (blue) and asthma (green). In the insert theQ0Attikon mutation confirmed by Sanger sequencing: sequencing results of proband compared with wild type and reference sequence. Translation initiation codon (Met1) is affected by this mutation (top of the insert figure). Three-generation pedigree of the proband. Numbers below individual symbols are age at inclusion in the study. Smoking status (pack/years) and levels of alpha-1 antitrypsin in serum (g/l) are also depicted. In details shown for each individual [parents I1, I.2, the proband, sister and brother II.1, II.2, II.3 from the third marriage of the father of the proband and children III.1, III.2 first marriage of the proband], the results of the analysis including LUMINEX (L), Isoelectric focusing (IEF) and gene sequencing (S). *The variant c.-428G>A; c.-10T>C found in individuals I.2 and II.3 seems to be like a M variant in the IEF. c.1A>G corresponding to Q0Attikonnewly described mutation is depicted at the proband II.1 in compound heterozygous state with PI*Z as well as at his sister II.3 and his children III.1 and III.2 at heterozygous state. (M/c.1A>G) for individual I.1 indicates an obligate carrier status. All members of the bloodline are reportedly asymptomatic except the proband which presents emphysema (red), bronchiectasis (blue) and asthma (green). In the insert theQ0Attikon mutation confirmed by Sanger sequencing: sequencing results of proband compared with wild type and reference sequence. Translation initiation codon (Met1) is affected by this mutation (top of the insert figure).](https://static.elsevier.es/multimedia/25310437/0000002900000006/v3_202311162326/S2531043723000934/v3_202311162326/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)



