Pleural effusion (PE) is a common clinical presentation of chronic kidney disease (CKD).1 Recently, two prospective observational studies evaluated the association of PE and mortality.2,3 One study revealed that the presence of bilateral and transudative PE was an indicator of increased mortality.2 The other study revealed that patients with PE caused by congestive heart failure and CKD in addition to bilateral PE had high mortality.3 Thus, we hypothesized that PE at the time of initiating maintenance hemodialysis is closely associated with poor outcome. We examined chest X-ray (CXR) images at the time of initiating maintenance hemodialysis to evaluate the association between PE and mortality.
This was a single-center, retrospective survey study. The local ethics committee of Hikone Municipal Hospital approved this study and waived off the requirement of obtaining written informed consent for all participants.
We reviewed all medical records of the patients who were started on maintenance hemodialysis at Hikone Municipal Hospital between January 2013 and December 2017. Patients who were followed up for a minimum of 3 months post initiation of maintenance hemodialysis or until death were included. Patients who had undergone peritoneal dialysis or had values missing from their medical records were excluded. Survival data were calculated from the time of initiating maintenance hemodialysis to time of death.
PE was assessed using CXR images <1 week before the initiation of maintenance hemodialysis. PE was considered to be mild if the costophrenic angle was blunt, moderate if the effusion occupied one-third to half of the hemithorax, and severe if more than half of the hemithorax was opacified.4
Continuous variables were compared using Wilcoxon rank-sum test and expressed as mean±standard deviation, whereas categorical variables were compared using chi-square test and presented as frequencies with percentage. Survival was compared using Kaplan–Meier plots and log-rank test. The multivariate Cox proportional hazards model was used for evaluating differences between survival and the following explanatory variables, age, heart disease, serum albumin level, and PE. All statistical analyses were performed using JMP® 10 statistical software package (SAS Institute; Cary, NC, USA); p<0.05 was considered to be statistically significant for all analyses performed.
We screened 88 patient records, of which 6 were excluded for following reasons; 4 were followed for <3 months post initiation of maintenance hemodialysis, 1 underwent peritoneal dialysis, and another had missing CXR image before the initiation of maintenance hemodialysis. A final total of 82 patients were included in our study.
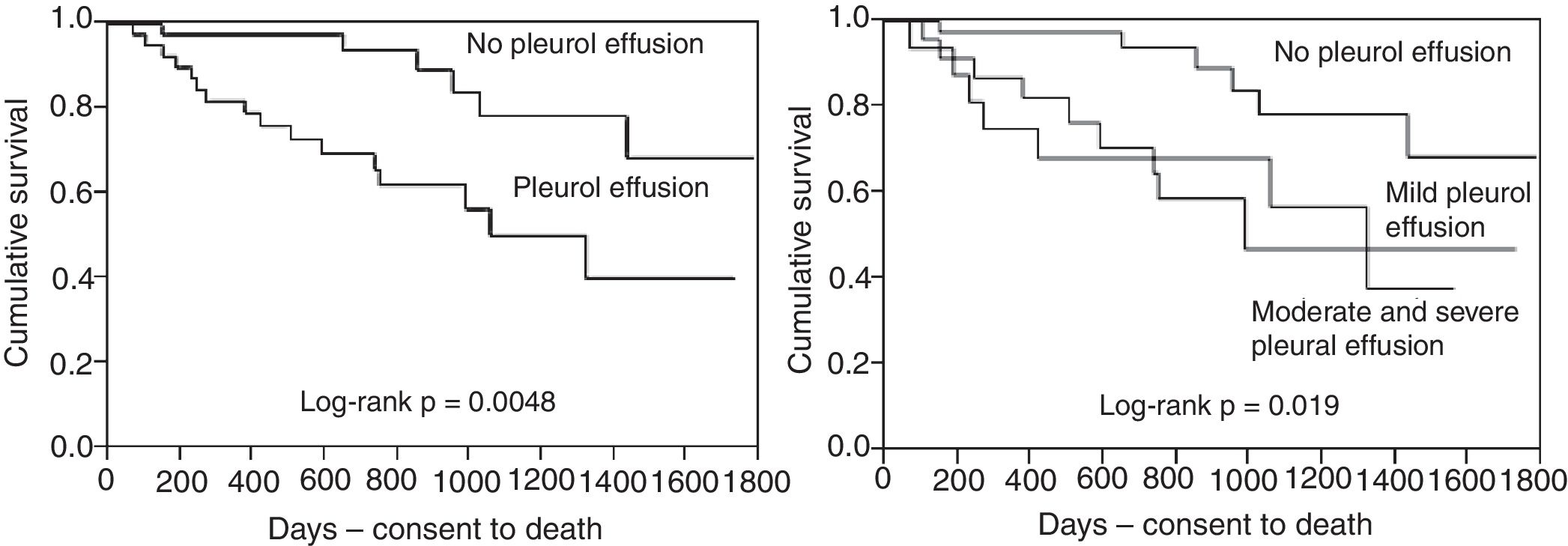
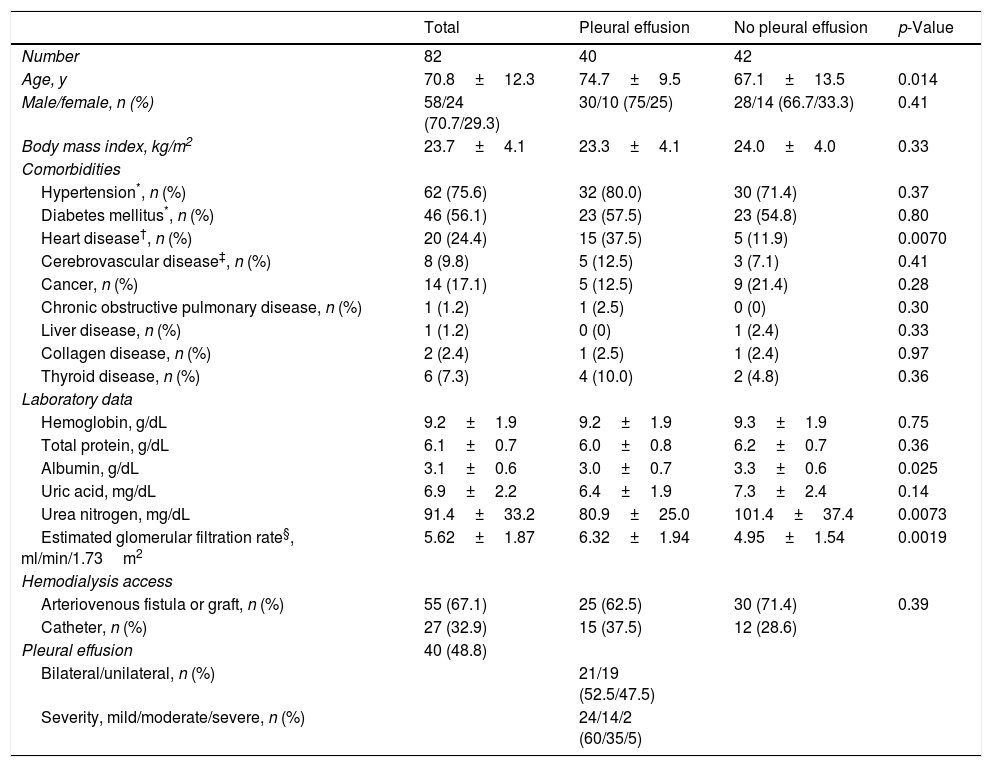
Clinical characteristics of study patients are presented in Table 1. The mean follow-up from the time of initiating maintenance hemodialysis was 765.6±475.4 days. The incidence of PE at the time of initiating maintenance hemodialysis was 48.8% (40/82 patients); PE was bilateral in 21 patients (52.5%) and unilateral in 19 patients (47.5%). Patients with PE were older and had a higher frequency of heart disease than those without PE. The presence of PE, regardless of its severity, was significantly associated with lower survival probability (Fig. 1). After adjusting for age, heart disease, and serum albumin level, the presence of PE was significantly associated with lower survival probability (hazards ratio: 2.78 [95% CI, 1.047–8.23, p=0.040]).
Clinical characteristics of study participants.
| Total | Pleural effusion | No pleural effusion | p-Value | |
|---|---|---|---|---|
| Number | 82 | 40 | 42 | |
| Age, y | 70.8±12.3 | 74.7±9.5 | 67.1±13.5 | 0.014 |
| Male/female, n (%) | 58/24 (70.7/29.3) | 30/10 (75/25) | 28/14 (66.7/33.3) | 0.41 |
| Body mass index, kg/m2 | 23.7±4.1 | 23.3±4.1 | 24.0±4.0 | 0.33 |
| Comorbidities | ||||
| Hypertension*, n (%) | 62 (75.6) | 32 (80.0) | 30 (71.4) | 0.37 |
| Diabetes mellitus*, n (%) | 46 (56.1) | 23 (57.5) | 23 (54.8) | 0.80 |
| Heart disease†, n (%) | 20 (24.4) | 15 (37.5) | 5 (11.9) | 0.0070 |
| Cerebrovascular disease‡, n (%) | 8 (9.8) | 5 (12.5) | 3 (7.1) | 0.41 |
| Cancer, n (%) | 14 (17.1) | 5 (12.5) | 9 (21.4) | 0.28 |
| Chronic obstructive pulmonary disease, n (%) | 1 (1.2) | 1 (2.5) | 0 (0) | 0.30 |
| Liver disease, n (%) | 1 (1.2) | 0 (0) | 1 (2.4) | 0.33 |
| Collagen disease, n (%) | 2 (2.4) | 1 (2.5) | 1 (2.4) | 0.97 |
| Thyroid disease, n (%) | 6 (7.3) | 4 (10.0) | 2 (4.8) | 0.36 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 9.2±1.9 | 9.2±1.9 | 9.3±1.9 | 0.75 |
| Total protein, g/dL | 6.1±0.7 | 6.0±0.8 | 6.2±0.7 | 0.36 |
| Albumin, g/dL | 3.1±0.6 | 3.0±0.7 | 3.3±0.6 | 0.025 |
| Uric acid, mg/dL | 6.9±2.2 | 6.4±1.9 | 7.3±2.4 | 0.14 |
| Urea nitrogen, mg/dL | 91.4±33.2 | 80.9±25.0 | 101.4±37.4 | 0.0073 |
| Estimated glomerular filtration rate§, ml/min/1.73m2 | 5.62±1.87 | 6.32±1.94 | 4.95±1.54 | 0.0019 |
| Hemodialysis access | ||||
| Arteriovenous fistula or graft, n (%) | 55 (67.1) | 25 (62.5) | 30 (71.4) | 0.39 |
| Catheter, n (%) | 27 (32.9) | 15 (37.5) | 12 (28.6) | |
| Pleural effusion | 40 (48.8) | |||
| Bilateral/unilateral, n (%) | 21/19 (52.5/47.5) | |||
| Severity, mild/moderate/severe, n (%) | 24/14/2 (60/35/5) | |||
Heart disease included congestive heart failure, peripheral artery disease or amputation, angina pectoris, and atherosclerotic heart disease.
The renal function was evaluated by estimating glomerular filtration rate using the following equation developed for Japanese patients; estimated glomerular filtration rate (ml/min/1.73m2)=194×serum creatinine−1.094×age−0.287 (×0.739, if female).7
PE at the time of initiating maintenance hemodialysis was associated with poor prognosis. Studies assessing the correlation between mortality and PE caused by CKD are limited. Kwan et al. retrospectively described that PE was associated with high mortality in patients undergoing maintenance peritoneal dialysis.5 DeBiasi et al. prospectively reported that patients with PE caused by CKD had high mortality.3 These data are consistent with our findings. However, the underlying association between PE and mortality remains unknown. Future studies exploring the underlying association between high mortality and PE at the time initiating maintenance hemodialysis are required.
In this study, the incidence of PE at the time of initiating maintenance hemodialysis was 48.8%. Two previous studies have shown that the incidence of PE was 6.7% in patients with CKD (stage 3−5) under pre-maintenance dialysis and 20.2% in those undergoing long-term maintenance hemodialysis.4,6 These differences could be influenced by the timing of evaluating PE.
This study has four major limitations. First, this single-centered study included only a small number of patients. Second, we did not evaluate PE by modalities other than CXR, including chest computed tomography and thoracic ultrasonography. Third, the cause of PE could not be determined because thoracentesis was not performed. Fourth, other prognostic factors, including parathyroid hormone levels and activities of daily living, were not evaluated.
We identified that PE at time of the initiation of maintenance hemodialysis was a common complication associated with poor prognosis. Future studies examining whether the initiation of maintenance hemodialysis before PE can enhance prognosis are required.
FundingThis study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflicts of interest to declare.
None.