The reversed halo sign (RHS) refers to a focal rounded area of ground-glass opacity surrounded by a more or less complete ring of consolidation.1 It was initially described by Voloudaki et al.2 in two patients with cryptogenic organizing pneumonia (COP). Zompatori et al. used the term atoll sign,3 while the term RHS was first introduced by Kim et al.4 Initially, it was considered to be relatively specific to COP. Subsequently, the RHS has been reported in a wide variety of pulmonary diseases including infectious, neoplastic and non infectious/non neoplastic, thus losing its specificity.
Despite the fact that the RHS is quite extensively studied in terms of radiology,5 data on corresponding pathology are actually scarce. In the initial report by Voloudaki et al.,2 histology from the periphery of the lesions showed the presence of buds of granulation-fibromyxoid tissue in the lumen of alveolar ducts and alveoli, while the central area of the ground glass attenuation showed less granulation tissue and more alveolar septal inflammation. These findings were confirmed in a much larger cohort of 43 patients, where the “ring” of the RHS corresponded pathologically to intraluminal organizing fibrosis in distal air spaces.6 The RHS has no specificity as it can be observed in a wide variety of diseases. Furthermore, it can appear in different phases of disease progression. The pathology remains vague as to what this image implies. In order to delineate the pathology correlation, it is essential to specify the actual timeframe at which the RHS appears. We present a representative case of radiation induced organizing pneumonia (RIOP) in which the temporal evolution of imaging findings sheds light on the pathological and clinical significance of the RHS.
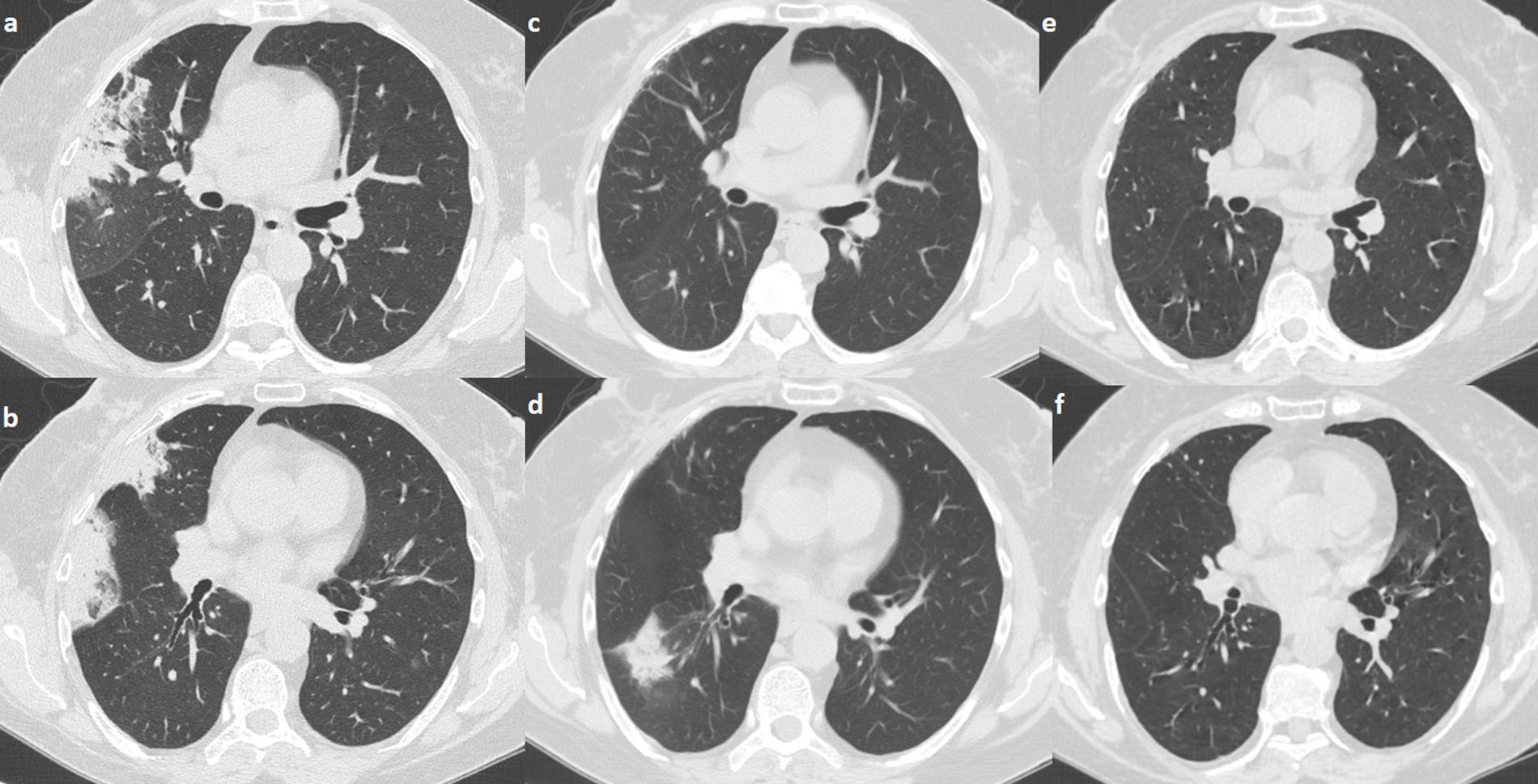
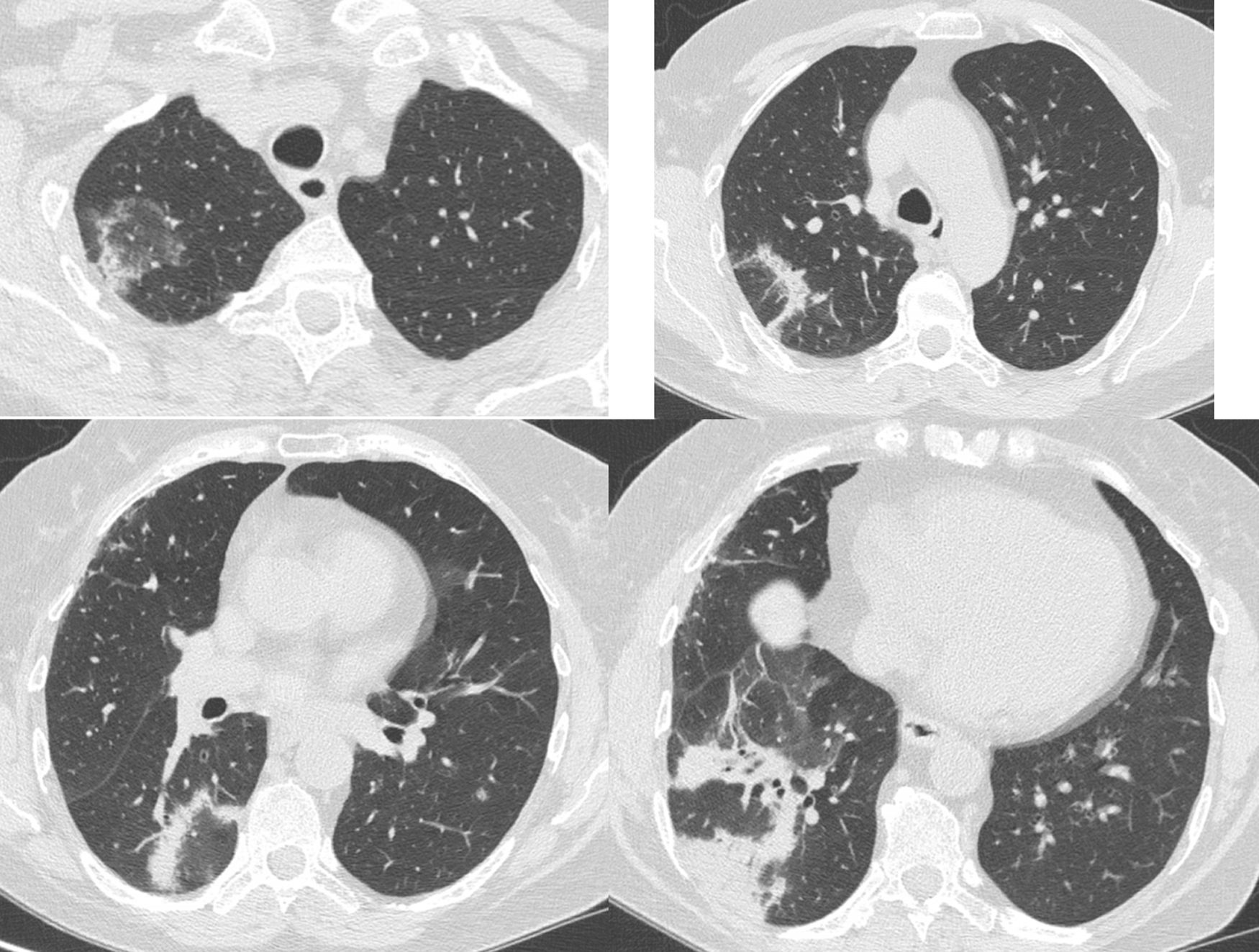
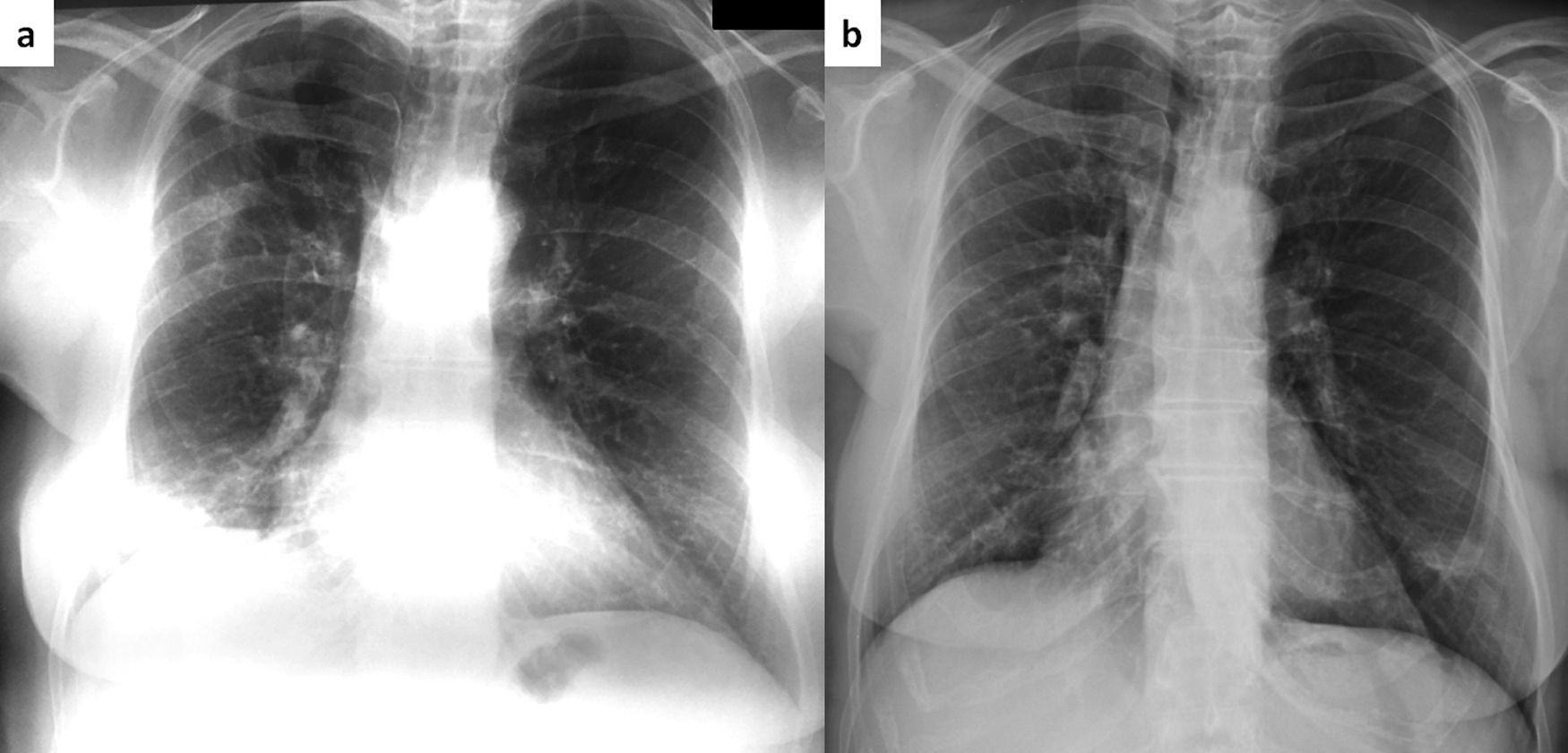
A 68-year-old female was diagnosed with right breast cancer. She was managed with breast conserving surgery (lumpectomy) followed by radiotherapy. The patient presented with progressive dyspnea and fatigue 16 weeks after completion of radiotherapy. CT revealed areas of consolidation in the right upper and right middle lobe (Fig. 1a,b), ipsilateral to the irradiated side. A presumptive diagnosis of radiation pneumonitis was made at an outside facility and the patient was started on corticosteroids with gradual tapering. A subsequent CT after 4 months showed a complete resolution of the lung opacities in the right upper lobe but appearance of a new consolidative area in the right lower lobe (Fig. 1c,d). Corticosteroid therapy continued and after 3 months all opacities completely resolved (Fig. 1e,f). After 5 months from corticosteroid discontinuation, the patient complained again of fatigue. At that time, she was referred to our interstitial lung disease unit. A new HRCT exhibited several areas of ring consolidation surrounding a central area of ground glass opacity in the right upper and right lower lobe (Fig. 2). Based on history, clinical examination and imaging findings, a diagnosis of relapsing RIOP was established.7 This time, azithromycin was administered to the patient at a dose of 250mg three times weekly. After 2 months of treatment, the patient reported resolution of her symptoms. A chest radiograph showed an almost complete resolution of the abnormal imaging findings as well (Fig. 3).The patient reports no symptoms and has a normal chest radiograph and laboratory work-up, at 1 year. It is worth noting that the administration of steroids in the context of RIOP is related to a higher rate of relapse.8
Areas of consolidation in the right upper and middle lobe. The consolidative area in the right middle lobe has a “bird nest” appearance (panel a, b). After 4 months under steroid treatment there is clearance of the infiltrates in the right upper and middle lobe. There is a new area of consolidation in the right lower lobe consistent with a migratory pattern (panel c, d). After 6 months under steroid treatment there is a complete resolution of the imaging findings (panel e, f).
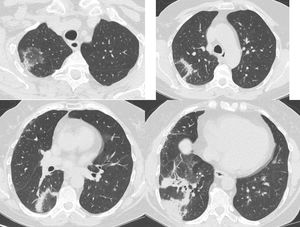
Same patient as Figure 1. Five months after treatment cessation. In the right upper and lower lobe there are areas consisting of an external rim of consolidation surrounding a central area of ground glass attenuation (reverse halo sign).
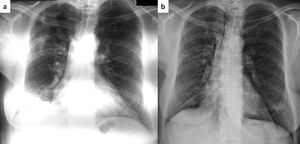
Same patient as Figure 1. Chest roentgenogram 5 months after cessation of steroids with areas of consolidation in the right upper and lower lung zone (panel a). After two months of treatment with azithromycin (250mg thrice weekly), there is a complete resolution of abnormal findings (panel b).
The incidence of organizing pneumonia (OP) following breast radiotherapy is 1–3% and the interval period between completion of radiotherapy and onset of symptoms is 2.3–47 weeks (usually 4–24 weeks).7–14 Increasing age (≥50 years),9,15 concurrent endocrine therapy,9 smoking,15 increasing lung volume within the radiation field16 and increasing central lung distance13,16 have been associated with the development of RIOP. Usually, the initial imaging findings are unilateral (ipsilateral to the irradiated side), however, bilateral involvement can also be seen at presentation.7,8,10 In patients with migratory infiltrates and relapsing disease, it is more likely to have bilateral involvement.10 The exact pathogenesis and pathology of RIOP has not been clarified. Proposed mechanisms are radiation induced injury of alveolar epithelial cells (DNA double-strand breaks) and mutations in the ATM (ataxia telangiectasia mutated) gene.17 The ATM gene plays a key role in the repair of DNA double-strand breaks.18 However, it is important to highlight that radiation in the lung causes endothelial damage and impairs the stability of the alveolar-capillary membrane.19 The increased permeability of the latter leads to the exudation of plasma protein, intra-alveolar clotting, deposition of fibrin, and finally colonization by matrix-producing fibroblasts resulting in the formation of intraluminal bus of connective tissue.
In order to understand the radiology-pathology correlation, reviewing diseases in which the RHS is the initial radiological manifestation of the healing response can provide significant insight. Such characteristic paradigms are pulmonary infarction, mucormycosis and pulmonary radiofrequency ablation. The study of underlying pathology in these cases reveals a common pathology and response to injury.
The RHS has been described in cases of pulmonary infarcts, consisting of a thick consolidative “ring” surrounding a central ground glass area. Pathologically, the external rim of consolidation corresponds to organizing granulation tissue associated with the presence of histiocytes, foamy macrophages, hemosiderin-laden macrophages, and activated fibroblasts. The central area corresponds to coagulative necrosis associated with hemorrhage. Necrosis often assumes a geographic pattern, crisscrossing the parenchyma and traversing interlobular septa.20 Fluorodeoxyglucose-positron emission tomography computed tomography (FDG-PET/CT) provides valuable information delineating the metabolic pathophysiology of the RHS in the context of pulmonary infarction. Specifically, in cancer patients with pulmonary infarction, the “rim sign” has been described.21 It corresponds to slight FDG uptake strictly along the periphery of the infarct, while the central area shows no uptake. This finding has a strong correlation with the pathological features of pulmonary infarction as the increased peripheral uptake is due to the increased metabolic activity of the activated fibroblasts and macrophages that are located in this area.
The RHS seems to be consistent in cases of pulmonary infarction regardless of the cause of interrupted blood flow. In the clinical setting of immunosuppression (especially neutropenic patients with hematologic malignancies receiving chemotherapy or bone marrow transplantation), the RHS is considered highly suggestive of underlying fungal infection, especially pulmonary mucormycosis.22,23 It is worth emphasizing that Mucorales infections are more angioinvasive than aspergillus infections,23 resulting in vessel thrombosis, thus infarction of infected tissues is a hallmark of the disease. The lesions are mainly peripheral in distribution.24 When the RHS is present in mucormycosis, the central ground glass area corresponds pathologically to coagulative necrosis, while the outer rim of consolidation to organization.25 From an imaging point of view, the RHS presents different morphological characteristics when present in OP and invasive fungal infections. The presence of reticulation within the central area, increased thickness of the outer rim exceeding 1cm (and the presence of pleural effusion), favors the diagnosis of the latter.26
Radiofrequency ablation (RFA) has been used for the management of selected patients with Non-Small Cell Lung Cancer (NSCLC) unable to undergo curative surgical resection, selected patients with pulmonary metastases or those with recurrences after surgery, chemotherapy, or radiation treatment.27 RFA leads to coagulative necrosis and, as expected based on the previous observations, it is a cause of the RHS with a “bird nest” morphology.28
In our case of RIOP, it is important to note that when relapse occurred, the patient sought medical advice almost immediately, thus imaging findings correspond to the initial phase of the healing process. Based on the paradigms of pulmonary infarction, angioinvasive mucormycosis and RFA, it seems that when the RHS is observed at the initial stages, it corresponds pathologically to activated fibroblasts encircling certain areas of lung injury. In the above mentioned entities, the initial lung injury results in coagulative necrosis. The actual nature of injury in cases of RIOP remains unknown. Intriguingly, radiation induced necrosis is also coagulative.
This type of response follows a specific course which is typically depicted by the resolution of a pulmonary infarction. Specifically, the resolution follows a centripetal course from the periphery to the center of the infracted area, which preserves its initial shape. On the other hand, there are cases where the clearance of an infiltrate starts from the center and expands centrifugally, creating an image consistent with the RHS.29,30 However, in such cases the RHS is not the initial radiological manifestation, it is observed during the healing phase and it does not have the pathophysiological significance as described above. Interestingly, Covid-19 represents an example where the RHS is observed at later stages of the disease, when consolidation develops around GGO or when the absorption of infiltrates starts from the center as mentioned before.31,32
From a pathological point of view, the presence of the RHS depends on a variety of parameters. The presence of low dense tissue in the central part (when necrosis, and/or edema is present) with dense (granulation) tissue in the surrounding area can manifest the RHS. Also, as mentioned earlier, there are cases when the healing process of OP starts from the central part. Finally, the presence of inflammatory aspects in the central area (NSIP like pattern, alveolar macrophages etc.) surrounded by a rim of granulation tissue can give the appearance of a lobular pattern or reversed halo sign.33,34
Given the wide range of clinical entities that can present with the RHS, it is important to determine the actual timeframe at which it occurs, i.e. at the beginning or during the response of the lung to injury. This can increase the clinical significance of the RHS by narrowing differential diagnosis. Treatment for OP is based on steroids. The recommended doses are significantly high35,36 and one of the main problems in the management of OP is not the disease itself but the management of steroid induced complications (e.g., obesity, osteoporosis, diabetes, depression, and immunosuppression). Based on the above observations, the RHS reflects the initial step of a successful response to lung injury. The type of injury is coagulative necrosis, regardless of the actual cause of reduced blood flow (e.g., thrombi in pulmonary embolism, fungal angioinvasion in mucormycosis, and thermal injury in case of RFA). In cases of RIOP, the RHS also seems to represent an initial response to injury. The actual cause of injury (parenchymal or vascular) is unknown. It is intriguing that radiation causes coagulative necrosis and impairs the permeability of the basement membrane leading to intra-alveolar clotting, deposition of fibrin, and finally colonization by matrix-producing fibroblasts resulting in the organizing pneumonia pattern. Equally intriguing is the fact that steroid treatment increases the recurrence of RIOP after breast-conserving therapy, thus prolonging disease duration.8 Given the fact that the RHS represents the initial phase of a successful healing response, it would be important to clarify the natural history of these cases without the administration of drugs. In conclusion, phenotyping cases of OP based on clinical and imaging characteristics, can lead to more specific management, avoiding unnecessary adverse events.
Conflicts of interestThe authors have no conflicts of interest to declare.